Endoscopy-assisted Vitrectomy For Severe Endophthalmitis
When initial VA is limited to light perception, better visualization can make a large difference, but early timing for surgery is crucial.
CLAUDE R. BOSCHER, MD
During the 2012 Retina Society meeting in October in Washington, DC, Harry W. Flynn, MD, gave the J. Donald M. Gass lecture: “Lessons from an outbreak of Streptococcus endophthalmitis after intravitreal injections of bevacizumab.”
Twelve patients presented within one to six days after injections, nine of them without any prior treatment for endophthalmitis.
Eleven of the patients had poor visual outcomes: five were enucleated, and two were eviscerated. Common features were cyclitic membranes and hemorrhagic retinal detachments. The most common identified germs were Streptococcus mitis and Streptococcus oralis. Dr. Flynn mentioned that he was not sure whether endoscopy-assisted vitrectomy would have saved the lost eyes.
During the 2002 combined Retina-Vitreous Society meeting in San Francisco, I presented, along with Raphaël A. Amar, MD, and Dan A. Lebuisson, MD, also of the American Hospital of Paris, a poster entitled “Endoscopy-assisted vitrectomy (EAV) for severe endophthalmitis with initial visual acuity limited to light perception.” Our poster featured a retrospective analysis of a consecutive series of 12 pseudophakic eyes operated on with endoscopy from September 1995 to July 2002.
This study had been designed to determine whether the use of an endoscope as the visualization means during vitrectomy would improve the beneficial results of conventional vitrectomy, as demonstrated in 1995 by the Endophthalmitis Vitrectomy Study in eyes with light perception only at initial presentation.
|
Claude R. Boscher, MD, is on the faculty of the American Hospital of Paris. She reports no financial interests in any products mentioned in this article. Dr. Boscher can be reached via e-mail at cboscher@wanadoo.fr. |

Figure 1. In this case, endoscopic control of the penetration of the instruments in the other sclerotomies was possible.
|
Click on links to view videos. |
Endoscopy-assisted vitrectomy was performed between 20 hours and six weeks (mean 13 days) after the onset of symptoms and one to 20 days (mean seven days) after intravitreal injection of antibiotics alone in seven eyes. Vitreous cultures identified Streptococcus (D and F) in four eyes, Staphylococcus aureus in three eyes, and Staphylococcus epidermidis in one eye, and Streptococcus D was positive in the conjunctival swab in one eye.
I present here our study in its original 2002 version, with a current comment added.
PURPOSE
Endoscopy allows for viewing in 360° through nontransparent media, and high magnification and a tangential approach to the anterior “zonular” vitreous base, involved in anterior posterior vitreoretinopathy and cyclitic membranes. External scleral indentation, which masks the real depth of the anterior vitreous base (AVB) and which is potentially problematic after recent anterior surgery, is not necessary.
The purposes of the study were: 1) to collect additional information about in vivo anatomical vitreoretinal conditions in severe endophthalmitis with LP-only initial visual acuity: and 2) to determine the specific capacities of EAV, in terms of viewing and AVB cleansing, to improve the results of EAV.
METHODS
A retrospective, nonrandomized, noncomparative, consecutive, interventional case series was undertaken, involving 12 pseudophakic patients (seven women and five men), ages 46 to 95 years (mean 77), who were operated on from September 1995 to July 2002.
The timing for vitrectomy ranged from 20 hours to six weeks (mean 13 days) after the onset of symptoms. EAV was performed as a primary procedure in five eyes, and one to 20 days (mean 7 days) after intravitreal antibiotic (AB) injections alone in seven eyes.
The follow-ups ranged from three to 55 months (mean 24 months). A fiberoptic endoscopic system, including a laser channel, and a flow control (peristaltic pump) vitrectomy machine were used. Gram staining, vitreous cytology analysis, and cultures were performed in all cases.
Simultaneous intravitreal AB injections were performed in four cases; there were no intravitreal steroid injections. All of the patients received systemic quinolones. Patient charts, operative reports, and video recordings were reviewed, and follow-up data were provided by the referring ophthalmologists.
RESULTS
Endoscopic Anatomical Findings

Figure 2. Example of very early vasculitis.
The microbiological results and primary endoscopic anatomical findings are shown in Table 1 (page 43).
Thickly constituted cyclitic membranes covering the pars plicata and the pars plana were found in nine (75%) eyes (Figure 1; Videos 1 and 2*). These membranes prevented direct visualization of penetration of the infusion line and vitreotome in six eyes (50%) (Video 3), prevented detection of anterior retinal displacement in two eyes, and caused ciliary detachment in six (50%) eyes.
Cyclitic membranes were found in constitution in three eyes as early as 20 hours after the onset of symptoms (Table 1, Case 3; Video 2), causing partial ciliary detachment in one eye.
Retinal detachment was present initially in four eyes (33%). Total rhegmatogenous RD with an equatorial flap tear was seen in one eye, with PVR A12 D2 and a basically avascular retina (empty vessel walls; no attempt was made to fix this RD).
Pre-existing partial peripheral tractional RDs were present, but not identified preoperatively, in two eyes. These RDs were transformed into mixed tractional-rhegmatogenous RDs by iatrogenic retinal perforation during penetration by instruments.
Extremely thick cyclitic membranes prevented the endoscopic identification of anterior ciliary-retinal displacements, inhibiting the advantage of creating sclerotomies under endoscopic viewing control (Video 3).
Also, a pre-existing tractional macular detachment was present in one eye.
Of 11 previously unvitrectomized eyes, intravitreal pus was concentrated anteriorly only in six of 11 eyes (50%) ( Videos 4 and 5). Posterior vitreous detachment was present spontaneously in four eyes, posterior vitreous schisis was seen in two eyes, and the vitreous was still attached in five eyes.
Pus pockets were found between the posterior hyaloid and the retina, in contact with the retinal surface, in four eyes, and were absent in one eye. Macular pucker with tractional macular detachment was present in one eye.
Regarding vascular involvement (Figures 2, 3, and 4), there was an aspect of subtotal arteriovenous occlusion in two eyes; central retinal vein occlusion was seen in six (50%) eyes, which was very severe, with macular infarction, in five cases (41%); and aspects of macular infarction were present in three of five eyes without preoperative intravitreal AB injections, as early as 20 hours after the onset of symptoms (Table 1, Case 3; Video 4).

Figure 3. Example of early vasculitis: probable spots of leukocyte thrombosis.

Figure 4. Detachment of the posterior hyaloid from the macular surface: severe perivascular involvement is visible underneath.

Figure 5. Control of the endoscopic sclerotomy port by switching sclerotomies. The endoscope is placed into the previous vitreotome’s sclerotomy port: tractional retinal tears and detachment under the endoscope’s port.
Extensive retinal ischemia alone, with disseminated preretinal hemorrhages up to the ora serrata, was seen in nine (75%) eyes (Video 5). Pus was found inside the posterior capsular bag in all 12 (100%) eyes (Video 3).
Intraoperative Complications
In two eyes (16%), there was retinal perforation at the entry site because of undetected anterior ciliary-retinal displacement (by the endoscope probe’s tip in one eye and by the infusion line in one eye).
Iatrogenic retinal tears occurred in four (33%) eyes, caused by traction at a distance in two cases, including one giant flap tear, by direct browsing during an attempt to achieve total vitreous base cleansing in one case (Video 6), under the sclerotomy during insertion and withdrawal of the instruments at the sclerotomy in two cases (Figures 5 and 6; Video 7), and during macular peeling in one case (Video 8).
Altogether, iatrogenic RDs were provoked by iatrogenic retinal perforation at the entry site in two (16%) eyes, and pre-existing partial tractional RDs were transformed into mixed tractional-rhegmatogenous RDs in three (25%) eyes (peripheral tears in two eyes, macular tear in one eye).
Follow-up
One eye was lost to follow-up immediately after EAV. The retina redetached at gas resorption in three eyes with gas tamponade; two of these eyes underwent reoperation with silicone oil injections, and the silicone could be successfully removed in one eye.
One eye developed pupillary block at one month after EAV. It was treated by YAG laser (visual acuity was recorded as hand motions). Total RD with no LP developed at five months, and the patient declined reoperation.
One eye developed neovascular glaucoma and was treated successfully with endoscopic cyclophotocoagulation (Figure 7). One eye developed recurrent inflammation with cystoid macular edema and was successfully treated with repeated vitrectomy with extensive endoscopic cleansing of the AVB and internal limiting membrane peeling.
One eye developed reopening of an ancient break previously treated with laser, with subsequent tractional/rhegmatogenous RD, cyclitic membrane, and hypotony. Reoperation was successful for the redetachment, and restoration of the intraocular pressure was progressive (one year).
Final retinal status was attached in eight of 11 eyes (72%). As noted, one eye was lost to follow-up, and silicone oil is still present in two eyes. Two eyes are phthisic (in one eye, repair of the RD was not attempted due to the endoscopic findings, and one eye redetached at five months, and reoperation was declined).
One patient might have developed unproven sympathetic ophthalmia one year after surgery. Five of 11 (45%) eyes achieved visual acuity ≥10/200, and one of 12 eyes (8%) achieved visual acuity of 20/32.
DISCUSSION
RD in Severe Endophthalmitis With VA Limited to LP Only
Our series, limited to eyes with the most severe initial visual impairment, had a high rate of preoperative RD (33%). There was a significant rate of intraoperative RD (16%) and of aggravation of a preoperative, nonidentified tractional RD into mixed tractional-rhegmatogenous RD (25%).
In Nelsen and colleagues’ series of endophthalmitis cases (not limited to LP at initial presentation),1 in which RD was reported in 21% of cases, 6% of the cases were intraoperative. According to our own endoscopic findings, we suspect, as in fact suggested by the authors themselves, that some of the RDs classified as postoperative in that series, and in others, might have been either nonidentified, intraoperative RDs or RDs caused by nonidentified, intraoperative retinal perforations/tears.
Risk-benefit Ratio of Achieved AVB Peeling
Leaving some vitreous in place carried a risk of secondary retinal detachment/cyclitic membranes/hypotony in one eye. AVB peeling proved hazardous in the specific indication of endophthalmitis with LP vision only at presentation.
Direct perforation occurred, during the penetration of the endoscope and of the infusion line, due to anterior displacement, which cannot always be controlled intraoperatively because of the specific density, elasticity, and depth of early cyclitic membranes.
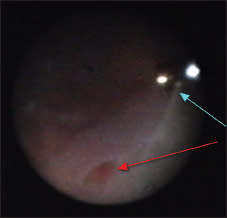
Figure 6. Iatrogenic tractional tear (red arrow) during insertion and withdrawal at the vitreotome’s sclerotomy port (blue arrow).
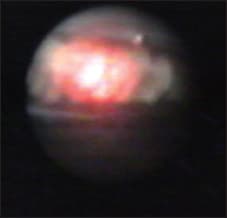
Figure 7. Endocyclophotocoagulation in a case of neovascular glaulation in a case of neovascular glaucoma.

Figure 8. Iatrogenic browsing of the pars plana during dissections with moderate bleeding (red arrow).
Induced traction occurred at a distance during core vitrectomy and during insertion and withdrawal at sclerotomy sites (Figures 5 and 6). This complication was unusually frequent compared to other indications for vitrectomy and was likely due to a decrease in normal retina-to-retina adhesion forces in endophthalmitis (potentially secondary to vascular abnormalities and ischemia induced by toxic involvement).
Attempts to achieve PVD and to dissect membranes, fibrin, and pus until reaching the surface of the retina and ciliary epithelium proved hazardous, with a risk of creating iatrogenic breaks (Figure 8).
In discussions and comments about the Endophthalmitis Vitrectomy Study,2 the limited character of vitrectomy in the protocol is rarely recalled. However, that protocol clearly specified that “if there was no posterior vitreous separation, no attempt was made to induce a vitreous detachment, and the posterior vitreous was not aggressively removed.” The rate of RD after vitrectomy was lower, but the specific rate in the subgroup with initial VA of LP only was not specified in the publication.
Primary Tamponade
Gas tamponade was insufficient in cases of RD secondary to severe endophthalmitis. One eye might not have been lost, and reoperation might have been avoided in two eyes if silicone oil had been the primary tamponade. Silicone oil should be the tamponade of choice.
Significance of Initial VA of LP Only
In all the eyes in our study, extremely severe damage was seen: pus invasion, early, strong, elastic cyclitic membranes; extensive vascular involvement; and retinal detachment.
During the same period, we performed EAV on three eyes with initial VA better than LP only, ranging from hand motions to 10/200 (Table 2).
This sample was too small to compare directly with that of the current study, but no organized cyclitic membranes, ciliary/retinal detachments, or slightly comparable vascular involvement were found in these eyes. Extensive ischemia with retinal hemorrhages up to the ora serrata was found in only one eye. Thus, initial visual acuity of LP only indicates RD, severe toxic damage, or both.
CONCLUSIONS
The specific viewing ability afforded by EAV allowed, in severe endophthalmitis with initial visual acuity of LP only, for visualization of the following:
► Extremely early development of elastic cyclitic membranes and early ciliary and anterior displacements with potential entry-site complications.
► A high rate of RD.
► Early extensive vascular involvement with aspects of macular infarction not imputable to AB toxicity.
► Early extensive anterior retinal ischemia, which can lead to secondary neovascular glaucoma.
► Adverse indications for vitrectomy.
On one hand, anterior vitreous base cleansing can be achieved as endoscopic visualization allows for it, to remove toxins and inflammation and to decrease the risk of postoperative RD. On the other hand, it is necessary to balance these benefits with the risk that extensive dissection might be iatrogenic, because of the specific features of endophthalmitis, including severe traction and friable ischemic retina.
| Recommendations for Eyes with LP Only VA at Presentation |
|---|
|
1. Ideally, preoperative B scan/ultrasound biomicroscopy should be performed, to reveal any RDs and ciliary and retinal anterior displacements. 2. Place the infusion line in the anterior chamber until endoscopic control of the pars plana is possible. 3. Use a 7-mm infusion line in the vitreous cavity. 4. Create sclerotomies 2.5 to 3 mm from the limbus. 5. Perform limited core vitrectomy with low flow control (1 cc per minute) to reduce traction at a distance. 6. Administer intravitreal steroids to protect against toxic damage. 7. Perform laser treatment of peripheral retinal ischemia to prevent neovascular glaucoma. 8. Use primary silicone oil tamponade in cases of RD. 9. Close follow-up (rubeosis, IOP, B scans) and prompt reoperation in cases in which membranes left in place (to avoid iatrogenic tears on friable retina) can induce secondary RD and/or hypotony. |
Our results show that despite the additional visualization provided with the endoscope, EAV sometimes could not overcome entry-site difficulties. Also, contrary to other indications, obstinately achieved anterior base cleansing can be harmful in severe endophthalmitis


Endoscopy-assisted vitrectomy in endophthalmitis requires training in the specific problems of both endoscopy (video control, orientation, manipulation of probe) and endophthalmitis (discrimination between vitreous membranes and ischemic avascular retina).
COMMENTS, OCTOBER 2012
Interestingly enough, we had emphasized in 2002 that endoscopy visualized severe lesions — retinal necrosis, cyclitic membranes, and RDs, which happened to be similar to those that Dr. Flynn and coauthors found on histopathologic examination during the 2011 outbreak.
From our endoscopic findings — as early as 20 hours after symptom onset — LP vision only at presentation meant a high probability of severe retinal necrosis, RD, or both. Over the last decade, our findings with endoscopy have remained identical.
In Bascom Palmer’s recent experience, such eyes were lost due to phthisis when not operated on, and the outcomes of conventional vitrectomy were poor. The Endophthalmitis Vitrectomy Study (EVS) found that vitrectomy was beneficial only for those eyes with LP only at presentation provided that the vitrectomy was limited, and no attempt was made to induce PVD surgically, in the cases in which PVD was not already present.
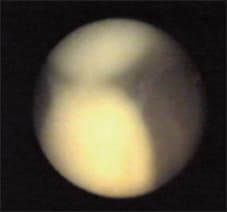
Figure 9. Disparity of vitreous viscosity: collected intravitreal pockets of pus.

Figure 10. High-magnification cleansing of the pars plana and pars plicata.
In our series, only two eyes were phthisic. The retina was attached in 72% of eyes (with silicone oil in two eyes), and 41% of eyes achieved measurable VA ≥10/200. Our results might have been even better if we had used silicone oil as the primary internal tamponade.
The EVS has been questioned over the past decade. Several arguments have been made in favor of “complete early vitrectomy.”3,4 Regarding the argument that surgical instrumentation has improved since the EVS was published, it does not apply to our series because we were always using flow-control vitrectomy, which compensates for vitreous viscosity disparities (Figure 9).
Our series demonstrates that the incomparable viewing capacity endoscopy provides, as well as the practice of flow control vitrectomy, may not always overcome the specific challenges of this peculiar indication of LP only VA at presentation.
Regarding retinal perforation at the entry site of the endoscope itself, the surgeon must understand that it can never be controlled at first. MVR blade perforation must be created under microscopy; then, what the surgeon sees on the video screen after the endoscope has crossed the sclerotomy tunnel are the tissues located right in front of the probe’s tip.
Regarding the other sclerotomies, established cyclitic membranes have a specific density and elasticity and may prevent viewing control of the second and third MVR blade perforations at the pars plana, masking potential anterior ciliary and retinal displacements (see Video 3).
In our opinion, the most important factor in EAV is the timing. Our experience in cases with LP-only vision, as well as in less severe cases — at the time of the original publication, as well as since then — has convinced us that “early complete vitrectomy” is indeed the most logical approach. Further, mastering endoscopy will undoubtedly yield improvements, both for the prognosis and for the management of endophthalmitis.
| Less Than 48 Hours After Onset of Symptoms |
|---|
|
1. Perform complete EAV, with dissection of the anterior part of the anterior vitreous base and cleansing of the capsular bag. Precautions include sclerotomies at 2.5 mm and an infusion line in the anterior chamber until endoscopic viewing control of the pars plana is possible. Early complete EAV should prevent RD, cyclitic membranes, and hypotony (Video 9). 2. Endophotocoagulation of the anterior ischemic retina will help to prevent secondary neovascular glaucoma. 3. Inject intravitreal dexamethasone, in addition to intravitreal antibiotics, after complete endoscopic dissection of the anterior vitreous base. 4. Prescribe high doses of vitamin D and vitamin C because of their actions against toxin-related inflammation, in addition to systemic AB. 5. Administer high doses of prednisone intravenously during or right after the primary procedure, in addition to systemic quinolones. |
| More Than 48 Hours After Onset of Symptoms |
|---|
|
1. EAV should consist of central vitrectomy only, with an infusion line in the anterior chamber and sclerotomies at 2.5 mm. 2. Steps 3 to 5 above should proceed, as in early cases. 3. Reoperate with endoscopy as early as the acute inflammatory phase is under control. By that time, the viewing advantage of EAV, with the capacity to perform cleansing of the anterior part of the ABV (Figure 6) and of the capsular bag, as provided by endoscopy, can be taken of with minor risks. However, reoperation should be undertaken very shortly thereafter (as early as 24 hours later) (Video 10). 4. Perform endophotocoagulation of the anterior ischemic retina to prevent secondary neovascular glaucoma. 5. Silicone oil should always be the tamponade of choice. |
However, VA of LP only at initial presentation in real life may have two different meanings, according to the timing of the initial presentation and the amount of time after the onset of symptoms of endophthalmitis.
In cases that present short time after symptom onset, LP-only vision indicates the extremely severe pathogenicity of the causal agent. This relationship is illustrated in our series by Case 3, which was operated on only 20 hours after the onset of symptoms and which presented early vasculitis, macular infarction aspect, and a cyclitic membrane already in constitution (Video 4). EAV was certainly the best option possible for a cure, yet extensive ischemia (which we did not treat preventively at that time) resulted in neovascular glaucoma one month later.
However, it should be noted that, despite this complication, the final VA (20/200) demonstrates that severe vascular involvement at the posterior pole is partially salvageable when vitrectomy is performed early (Video 4).
In cases in which presentation is delayed — in our experience, 48 hours or more after symptom onset, including all the more those patients who have already received intravitreal AB as primary care — LP-only vision indicates that extremely deleterious consequences are already present, in terms of retinal fragility and anterior tractional membranes.
In our series, iatrogenic complications occurred in cases operated on between eight days and 51 days after symptom onset. In these cases, at least in our study, even if the prognosis seemed better with EAV than the spontaneous prognosis or the prognosis provided by conventional vitrectomy, extensive vitrectomy — that is, vitrectomy actually allowed by the viewing capacity of endoscopy — carries a significant risk of iatrogenic damage ( Videos 3, 6, 7, and 8). This risk is not related to the use of endoscopy itself, but endoscopy cannot rule out the risks associated with any vitrectomy in such severe cases.
Therefore, our current recommendations for EAV in endophthalmitis with LP-only vision at presentation vary according to the time after the onset of symptoms.
CONCLUSION
Just as in 2002, we emphasize that surgeons must be experienced in both endophthalmitis and endoscopy to approach such cases, which are never candidates for training in endoscopy. We have been performing EAV since 1994 in all indications in which the vitreous base in involved (RD, proliferative diabetic retinopathy, trauma), and our overall experience is now more than 4,000 cases.
Severe endophthalmitis with LP-only vision undoubtedly remains, along with some cases of severe trauma, the most challenging type of case to treat with EAV. RP
| Instructions For Viewing the Videos Online |
|---|
|
To view the videos associated with this article, please view the article online or visit www.youtube.com/user/RetinalPhysician1. |
REFERENCES
1. Nelsen P, Marcus A, Bovino G. Retinal detachment following endophthalmitis. Ophthalmology. 1985; 92:1112-1117.
2. Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479-1496.
3. Kuhn F, Gini G. Ten years after… are findings of the Endophthalmitis Vitrectomy Study still relevant today? Graefes Arch Clin Exp Ophthalmol. 2005;243:1197-1199.
4. Gini G. Facing endophthalmitis: a European perspective on the EVS study. Retin Physician. 2008;5(8):47-50.








