Macula 2012: Highlights From the Conference
The annual East Coast conference on macula science and care returned to New York, offering new research and discussion on a range of topics.
Andrew E. Mathis, PhD, Medical Editor
Macula 2012, along with the annual meeting of the Atlantic Coast Retina Club, was held at the New York University School of Medicine earlier this year, providing a lively forum for discussion of the latest research and innovations in retina.
Sponsored by NYU and the North Shore–LIJ Health System and presented by the Manhattan Eye, Ear and Throat Hospital, with support from the Macula Foundation, the meeting featured first-day afternoon sessions on surgery, with the second day providing attendees with topics in a variety of areas, including hereditary disease, imaging, diabetic eye disease, RVO, AMD and uveitis. In addition, the morning of the first day featured the very popular ACRC case studies, with animated discussions of puzzling disease etiologies. The recaps provided here cover several aspects of surgery, as well as an enlightening second-day presentation on what drusen are and our evolving knowledge of the role they play in macular disease.
PETER SZURMAN ON RETINAL SURGERY
A compelling session on vitreoretinal surgery featured two lectures by Peter Szurman, MD, PhD, who is head of experimental ophthalmic surgery at the Institute for Ophthalmic Research of the University Eye Hospital in Tübingen, Germany. Dr. Szurman's first talk was on strategies for managing mass subretinal hemorrhages, which he described as “one of the last surgical challenges in AMD surgery.”
Weighing the options in cases of mass subretinal hemorrhage, Dr. Szurman first noted that waiting is not an option because the prognosis is so bad in AMD cases, although not quite as bad in non-AMD cases. Therefore, he reviewed the five most important strategies for removing blood and restoring vision.
First, Dr. Szurman discussed subretinal clot extraction, which he noted has not yielded any benefits over the natural course of AMD, particularly due to a high complication rate. Here, he referred to results from the Subretinal Surgery Trial, which found that any benefits from surgery disappeared by 12 months after enrollment.
The next strategy Dr. Szurman discussed was full macular translocation (FMT). In Dr. Szurman's own practice's experience with this procedure, patients with hemorrhages experienced stabilization with mild visual improvement. In certain cases, he noted, the improvements in visual acuity were quite impressive (Figures 1 and 2). Further improvements were limited by the complication rate.
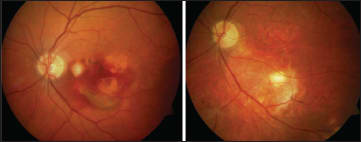
Figure 1. This patient's vision was counting fingers (left), but after FMT, logMAR VA of 0.125 was recovered (right).

Figure 2. This patient's vision was logmar 1/35 (left), but after FMT, logMAR VA of 0.32 was recovered (right).
Thus, Dr. Szurman explained, his practice's goal was, in part, to improve the complication rate. To achieve this goal, they undertook choroidal patch translocation (Figure 3). The procedure allows for the harvesting of very large grafts, which can then be used after the removal of CNV.

Figure 3. In some cases, choroidal patch translocation was necessary (images run from upper left to lower right).
Dr. Szurman described this technique as anatomically “wonderful,” noting that at one year, the healing process was well under way (Figure 4), with partial restoration of central fixation.

Figure 4. In this choroidal patch patient, partial restoration of central vision was under way at one year.
However, there are certain complications with this procedure. For instance, there is the tendency for fibrotic rings to form, and the visual acuity outcomes also have left much to be desired (Figure 5). Treatment using more minimally invasive techniques may help in such cases.

Figure 5. In cases such as this one, poor visual prognosis was due to the formation of fibrotic rings.
These minimally invasive techniques include pneumatic displacement with intravitreal recombinant tissue plasminogen activator (rTPA). Dr. Szurman described this technique as easy to perform with 23-gauge vitrectomy systems (Figure 6). He also noted the importance of avoiding air in the subretinal space.

Figure 6. Retinal surgeons can use 23-gauge vitrectomy for pneumatic displacement with rTPA.
With intravitreal rTPA, there are still adverse events, including profibrosis. Again, to attempt to lower the complication rate, Dr. Szurman's practice has been combining subretinal draining with intravitreal rTPA (Figures 7 and 8).

Figure 7. In this patient, subretinal drainage after rTPA injection resulted in postop VA of 20/100 from logMAR 0.05.
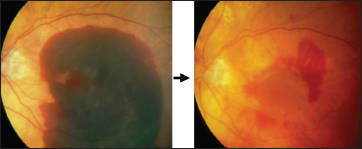
Figure 8. In this patient, subretinal drainage after rTPA injection resulted in postop VA of 20/200 from hand motions.
Here, Dr. Szurman transitioned to his second presentation, on new concepts in retinal reattachment surgery. One might assume, he said, that there might have been huge changes in this field. However, the basic principles remains unchanged: find the break, seal the break, and reattach the retina. Dr. Szurman undertook to discuss and explain what he identified as controversies in retinal reattachment surgery.
The first controversy concerned scleral buckling. On this point, he referred to the SPR study conducted in Europe in the last decade, from which he learned that scleral buckling results in comparable success rates and better visual acuity results in phakic eyes, while in pseudophakic eyes, the opposite outcomes are true. Given these results, Dr. Szurman stopped buckling pseudophakic eyes entirely.
The second controversy regarded gauge size and whether 20-gauge vitrectomy is safer than smaller gauges for retinal detachment surgery (Figure 9). Again, Dr. Szurman appealed to a study that found that success and complication rates were the same with both gauge sizes.
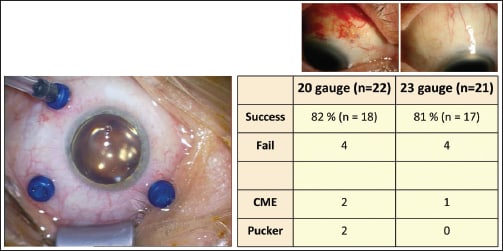
Figure 9. Success and complication rates were largely similar between 20- and 23-gauge vitrectomy.
The third controversy concerned whether trocar-assisted vitrectomy is safe and whether it can lead to insufficient tamponade. The study referenced here was one that compared 20-gauge and smaller-gauge vitrectomy, and this study found that trocar-assisted vitrectomy was safer (Figure 10).

Figure 10. Another study concluded that trocar-assisted small-gauge vitrectomy resulted in fewer complications.
The fourth controversy concerned whether silicone oil should be used if retinal breaks cannot be located. Here, Dr. Szurman urged surgeons to look harder for holes and recommended the use of subretinal Brilliant blue dye (Figure 11) to locate them.

Figure 11. Brilliant blue dye can be used to facilitate the finding of small holes.
The fifth issue was whether complete tamponade is impossible, and Dr. Szurman said that it is to date. The reason is that all tamponades available on the market are hydrophobic (Figure 12) with high surface tension, so physically it is not possible to avoid a small amount of water, leading to PVR. The hope that heavy silicone oil would obviate this problem, but it has not.

Figure 12. All of the tamponades currently available on the market are hydrophobic, resulting in incomplete tamponade.
If the only way to avoid superior PVR is complete tamponade, then, Dr. Szurman said, the question is whether complete tamponade will ever be possible. Here, he discussed work on a hydrogel and crosslinked synthetic copolymer tamponades in his own practice, as well as noncrosslinked and crosslinked natural polysaccharides (Figure 13).

Figure 13. Non-crosslinked natural polysaccharides are one type of hydrogel that may result in complete tamponade.
The last issue that Dr. Szurman addressed was whether or not his practice's attempts have been for naught because tamponade will no longer be necessary. He said that in simpler cases, increasing evidence is indicating that tamponade is not necessary. However, more research is needed.
CHRISTINE CURCIO ON DRUSEN
A highly interesting presentation during the Saturday afternoon session was given by Christine A. Curcio, PhD, who is on the faculty of the Department of Ophthalmology at the University of Alabama School of Medicine.
In her talk, “Drusen and the Oil Spill in Aging Bruch's Membrane,” Dr. Curcio began with a review of the published data on the relationship between basal linear deposits (BlinD) and soft drusen, both of which are associated with the AMD process.
Dr. Curcio pointed out that the available data indicate that BlinD and drusen are two forms of one lesion (Figure 14), and further that a soft druse-like material can be found in multiple compartments of the retina (Figure 15).

Figure 14. BlinD and drusen seem to be two forms of the same lesion.

Figure 15. A soft, druse-like materials can be found in several compartments of the retina.
A paper dating back to 1854 reported that drusen contained fat globules, and Dr. Curcio noted that oil red-O binding lipid, later shown to include esterified cholesterol, was identified as a constituent of drusen in 1962. Later studies including Dr. Curcio's own work over the last decade have established that the single largest component of drusen is esterified cholesterol and phosphatidylcholine, and that cholesterol is abundant in both drusen and BlinD. Meanwhile, 40 years of medical literature has also shown no consistent relationship of AMD with plasma cholesterol or lipoproteins, although AMD is associated with certain cholesterol-processing genes.
“So, what does all of this mean?” Dr. Curcio said by way of presenting the overall model of her presentation, ie, that lipoprotein particles form an “oil spill” in Bruch's membrane (Figure 16), which Dr. Curcio said was “just like an oil spill on the surface of sea water.” She demonstrated that these particles that appear to be vesicles on conventional post-fixation are actually solid particles when examined by quick-freeze deep etching. As age increases and lesions form, there is a fusion and pooling of lipids.

Figure 16. By conventional postfixation (top) or quick-freeze deep etch (bottom), lipoproteins resemble an oil spill.
The macula is the primary place where these particles accumulate; they fill in toward the RPE and form a layer on Bruch's membrane's inner surface — like an oil spill. Soft drusen are found only on the macula, and these drusen are made of the same material as BlinD.
Dr. Curcio then turned to the biochemistry of these lipoprotein deposits. The source of the esterified cholesterol is apolipoprotein B-containing lipoproteins that are secreted by the RPE. The RPE expresses the abetalipoproteinemia gene, which is required for lipidating apolipoprotein B lipoproteins. Among other functions, RPE cell lines secrete apolipoprotein B and solid esterified cholesterol-rich particles in culture. The fatty acid profile of these lipoprotein deposits suggests that diet is the source of fatty acids, Dr. Curcio explained. They are high in linoleate, like plasma lipoproteins, and low in DHA, unlike the outer segments. The source of cholesterol is unknown, and although endogenous synthesis is possible, the most intriguing possibility is that it comes from outer segments. Dr. Curcio continued by explaining that this oil spill has functional con sequences, as esterified cholesterol is correlated with high hydraulic resistivity and poor transport across Bruch's membrane. Lipoprotein constituents interact with free radicals, generating pro-inflammatory and pro-angiogenic compounds. Further, biomechanically unstable BlinD is a cleavage plane for ingrowing neovessels.
At this point in her presentation, Dr. Curcio turned to answer the question of what the oil spill means for the retinal physician treating AMD. While she cautioned that it will be some time before treatments based on our knowledge of drusen are available, she suggested that such treatments, as well as imaging technologies based on this knowledge, will eventually become available.
One way to clean up an oil spill, Dr. Curcio said, is to use dispersants (Figure 17). These would be analogous to bioremediation agents, such as detergents, surfactants, encapsulation lipases and apolipoprotein mimetics.

Figure 17. Dispersants could be used to remove drusen in the same manner that they are used in oil spills.
The next method is a “Top Kill” approach to limiting apolipoprotein B particles from the RPE (Figure 18). These approaches include methods of targeting hepatic VLDL, a precursor to LDL, including intraocular delivery of statins. Finally, the “Bottom Kill” approach would consist of addressing the problem through diet (Figure 19).

Figure 18. Apolipoprotein B molecules can be limited using a “top kill” approach, again like with an oil spill.

Figure 19. The “bottom kill” approach would consist of addressing the problem through diet.
The last part of Dr. Curcio's presentation addressed imaging technologies. Retinal physicians can learn from cardiologists who use imaging to monitor plaques and from geologists who watch oil spills. In the future, she said, the ability to image drusen might be likened to seeing tar balls as a result of an oil spill. In this way, doctors can know the “true burden of AMD's sight-threatening lesions — soft drusen and BlinD.”
WILLIAM AYLWARD ON RETINAL DETACHMENT SURGERY
The final two presentations of Macula 2012 were given by G. William Aylward, MD, who is a consultant vitreoretinal surgeon at the Moorfields Eye Hospital in London. In the first, entitled “New Ideas in Retinal Reattachment Surgery,” he began by noting that retinal detachment is a problem that still requires a solution, as the proportion of eyes that remained attached is not 100%. “Usually,” Dr. Aylward said, “this is because of untreated breaks.”
To solve this problem, Dr. Aylward continued, the surgeon needs to find the breaks. But what if the surgeon cannot? On one hand, he said, the surgeon can make an educated guess. However, he noted, in the case of subretinal detachments, this process will not help much. On the other hand, the surgeon can cover all of his/her options, including the use of encircling buckles, 360º laser, and silicone oil. Nevertheless, Dr. Aylward noted, this approach can be “heavy-handed,” particularly in the case of a single, tiny break.
How common are such breaks? Dr. Aylward said with internal searching during vitrectomy, one study found no breaks in 5% of cases. How ever, that figure predated the introduction of wide-angle viewing systems. Citing Stanley Chang, MD, Dr. Aylward mentioned that if the surgeon uses heavy liquid to the posterior pole (Figure 20) subretinal fluid will be driven through any breaks.

Figure 20. If the surgeon uses heavy liquid to the posterior pole, subretinal fluid will be driven through the breaks.
Next, he discussed the use of dyes. Citing a 1933 paper itself cited in 1967 in Duke Elder's System of Ophthalmology, Dr. Aylward noted that fluorescein had been used to identify retinal breaks. He recounted how he also tried fluorescein at the suggestion of one of his fellows a decade ago. This attempt was unsuccessful.
When trypan blue dye was introduced, Dr. Aylward said, he tried using dye again and found that it worked far better. Injecting trypan blue into the subretinal space with a 41-gauge cannula resulted in a plume that he described as having a “thumbs-up” shape. This approach, he said, “not only tells you where the break is, but where the break isn't.”
In presenting some outcomes from his practice, Dr. Aylward noted that potential problems do exist. For example, the chance of retinotomy is small but does exist, and toxicity is also an issue. Regarding the latter issue, he noted that the low concentration of dye used in humans, as well as the short duration of exposure, is not of tremendous concern. Also, lower-concentration dyes or brilliant blue dye can be used. Further, because far greater exposure time elapses when staining of the fovea for ILM peeling, Dr. Aylward argued that minimal exposure of the subretinal fluid is mild in comparison (Figure 21).

Figure 21. Minimal exposure of the subretinal fluid to dye is mild in comparison to that used in ILM peeling.
Concluding the first presentation, Dr. Aylward stated that staining of the subretinal fluid is an occasional but potentially useful technique for the treatment of retinal detachments.
Turning to his second presentation, on face-down positioning following retinal detachment, he said many retina surgeons, in cases of detachment, believe that fluid is leaking through retinal breaks more quickly than the RPE can pump it out. Thus, what surgeons believe they are doing is sealing the break using a gas bubble so that it can be permanently sealed with retinopexy (Figure 22).

Figure 22. Surgeons believe they are sealing a break with a gas bubble, but retinopexy can permanently seal them.
However, this belief has consequences, Dr. Aylward said, particularly for inferior breaks, where there is a greater tendency to use scleral buckles. Among these implications are that contact is required between the hole and the bubble, positioning is essential for inferior breaks, and failures occur due to poor positioning. “Therefore,” he said, “humans being humans, you can expect a lot of failures to occur.”
Some patients take positioning very seriously, but in general, achieving compliance is difficult, with some studies measuring it at less than 50%. To demonstrate, Dr. Aylward presented a case of a 56-year-old man, whom he described as having the “worst OCD of any patient I'd ever seen.” This patient had a left retinal detachment with a large tear at four o'clock, but he mistakenly positioned himself with his left cheek to the pillow. Nevertheless, upon examination, his retina had reattached.
Dr. Aylward then explained that if the gas really does have to be in contact with the break, more failures would be expected than actually occur. Furthermore, redetachments from posturing failures would be easier to detect.
“What I'm trying to do is build a case to suggest that this model is wrong,” Dr. Aylward continued. Among possible factors are the roles of fluid currents and shear stress from eye movements and head movements. Notably, the shear stress from head movements is twice as much as from eye movements. Further, there is an inverse correlation between the size of the gas bubble used and the shear stress in the eye (Figure 23).

Figure 23. There is an inverse correlation between the size of the gas bubble used and the shear stress in the eye.
In conclusion, Dr. Aylward stated his belief that gas bubbles may have an alternate mode of action other than tamponade and that, therefore, postoperative positioning may not be necessary.
RICK KAISER ON PVR PREVENTION
Backtracking a bit, one of the first presentations of the conference was given by Richard S. Kaiser, MD, of Mid-Atlantic Retina and the Retina Service of the Wills Eye Institute in Philadelphia. Dr. Kaiser presented data on the use of isotretinoin, also known as Accutane, for the prevention and treatment of proliferative vitreoretinopathy.
Dr. Kaiser began by discussing the causes, incidence and pathogenesis of retinal detachments. Turning to their natural history, he said the prognosis of retinal detachments was “typically dismal,” adding, “Surgery is our only option.” However, he continued, Accutane “might be an alternative for us.” It is prescribed for cystic acne but was actually developed as a chemotherapeutic agent. Furthermore, having been developed from vitamin A, it is a retinoid.
Retinoids are concentrated in RPE cells, where they play the role of inhibitors of RPE proliferation and migration. “So you can theorize,” Dr. Kaiser said, “that where the RPE is depleted of retinoids after a retinal detachment, the reduced concentration may contribute to loss of the inhibitory control over the RPE.” Thus, the RPE can proliferate and differentiate, forming a PVR.
At this point, Dr. Kaiser turned to previous studies of Accutane in humans. In a 1995 study, Fekrat et al. reported a high rate of retinal reattachment in 10 patients with PVR. Thirteen years later, Chang et al. reported a 93.8% reattachment rate over eight weeks in patients prospectively treated with Accutane vs 63.2% in the control group. “If you comb through the literature,” Dr. Kaiser added, “that's about the rate that you find of reattachment of PVR retinal detachments.”
Next, Dr. Kaiser discussed the side effect profile of Accutane, which he described as “vast.” In addition to being a teratogen, it can cause dryness of the skin, lips, mucus membranes and eyes, alopecia and elevated liver enzyme levels. Patients must also be watched for depression. Further, high doses of Accutane and long exposure periods have been linked slightly with a higher risk of developing ulcerative colitis. This last adverse effect has resulted in several class-action lawsuits. That being said, Accutane when used in low doses in acne patients has had a greatly reduced side-effect profile.
At this point, Dr. Kaiser moved on to the Wills Eye study of Accutane. The hypothesis for the study was that low-dose Accutane may be an effect surgical adjuvant in both preventing and treating PVR. To test this hypothesis, Wills launched the DELIVER study, which was a prospective, nonrandomized cohort study with consecutive, age-matched, pathology-matched, retrospective control group.
The study had two arms: one arm of 60 patients with recurrent retinal detachments with PVR, and the other arm of 60 patients at high risk for PVR. High risk was defined as preoperative PVR of grade B or worse, a retinal break >3 disc diameters, a retinal detachment of three or more quadrants, duration of detachment of >1 month, or a retinal detachment associated with trauma. The patients were adult men and postmenopausal women no older than 70 years old.
Dr. Kaiser shared the preliminary results of the study, which appear to be quite encouraging. He urged everyone to stay tuned for the final results, which should be available in early 2013.
CONCLUSION
Next year's meeting will be in Baltimore, so be sure to check the Events Calendar section in forthcoming issues of Retinal Physician for the announcement of the dates. RP








