Treatment Options for Submacular Hemorrhage
Decisions must be made on a case-by-case basis.
Robert J. Lowe, MD • Richard B. Rosen, MD • Ronald C. Gentile, MD
Submacular hemorrhages can cause devastating visual loss. They typically occur in elderly patients with exudative age-related macular degeneration, macroaneurysms or polypoidal choroidal vasculopathy, and in all populations in cases of trauma.1 Unfortunately, submacular hemorrhages usually have a poor visual prognosis if left untreated. This prognosis is due to several factors, including mechanical damage to the photoreceptors by fibrin infiltration. Fibrin infiltration between the inner and outer segments of photoreceptors can result in destructive shearing of the cells.2,3 Additionally, iron and hemosiderin liberated from hemolysis can have direct toxic effects on photoreceptor function.1,2 If the subretinal clot is too thick, diffusion of nutrients from the choroid to the photoreceptors can be impaired.2 Eventually, without treatment, a submacular fibrotic scar forms, especially in patients with AMD, which permanently limits any hope for visual recovery.1,2
How much vision is recoverable with treatment of submacular hemorrhage depends on the nature of the injury and the pre-existing macular function. Damage to the retina can occur as early as 24 hours.1 Overall, the window of opportunity for successful recovery is thought to be within the first two weeks of onset.3
PROGNOSIS AND TREATMENT
Patients with an otherwise healthy retinal pigment epithelium and photoreceptors will recover the most visual function. This is often the case for patients with submacular hemorrhage due to idiopathic choroidal neovascularization, retinal arterial macroaneurysms, polypoidal choroidal vasculopathy or trauma.2 Typically, traumatized eyes are usually healthy prior to the injury and have the best visual potential if rescued in time.4 However, prognosis is often poor in cases of advanced AMD due to underlying RPE disease, despite successful displacement of the hemorrhage.2
Spectral-domain ocular coherence tomography has proved to be invaluable in the diagnosis and management of submacular hemorrhages. It often helps guide the surgeon in deciding the best surgical option. Subretinal hemorrhages may be amenable to tissue plasminogen activator (TPA) and pneumatic displacement while sub-RPE hemorrhages may only benefit from pneumatic displacement alone.5 It is difficult to know whether TPA will diffuse sufficiently into the sub-RPE space to have a significant effect on hemorrhages in this location.
Given the poor prognosis of untreated submacular hemorrhages, several treatment strategies have been attempted. In the 1980s, traditional approaches to subretinal hemorrhage involved surgical extraction of the formed or liquefied clot.1,2 Intraocular forceps were used to extract the clot. These maneuvers risked damage to the underlying RPE as well as the photoreceptors. Visual outcomes were often disappointing because the retinal pigment epithelium and photoreceptors tended to be removed along with the clot due to the tight adherence of the RPE to the hemorrhagic clot.2 Large areas of RPE atrophy and retinal detachments resulting from the large retinotomy (30% to 37%) were common.1 Subsequent studies including the Submacular Surgery Trial suggested that subretinal extraction of hemorrhage in AMD produced equivocal results compared to observation.1,2
Poor visual improvement following submacular surgery stimulated the development of techniques designed to displace the clot rather than remove it.3 The introduction of TPA offered surgeons a new tool for submacular hemorrhage management. TPA is a protease enzyme that converts plasminogen into plasmin. Plasmin, in turn, can degrade the fibrin clot. In the early 1990s, surgical pneumatic displacement with TPA allowed the use of a smaller retinotomy.1 A pars plana vitrectomy (PPV) would be performed, then subretinal TPA was injected through the retina using a 39- or 41-gauge needle to create a focal retinal elevation. Clot dissolution took 15 to 60 minutes, which necessitated a break in the surgical procedure.2 The liquefied clot was then removed during the subsequent air-fluid exchange. Perfluorocarbon liquid could also be used to help squeeze the liquefied clot through a retinotomy. The technique of injecting the TPA subretinally and removing the blood via air-fluid exchange was felt to be less disturbing to the photoreceptor and RPE architecture.2 Not having to manually extract a formed clot reduced the need for a large retinotomy.2 This helped limit the risk of surgical damage to the RPE and photoreceptors.2

Figures 1 A-G show the pre- and postoperative appearance of a submacular hemorrhage in an 88-year-old hypertensive woman secondary to a macroaneurysm. The patient was treated with pars plana vitrectomy, subretinal TPA and air-fluid exchange with 20% SF6. (A) Fundus photo of 88-year-old woman with recent submacular hemorrhage. (B) Presurgery FA of submacular hemorrhage. (C) Presurgical OCT of submacular hemorrhage. (D) Postsurgical OCT of submacular hemorrhage. (E) Postoperative month one: OCT through the macroaneurysm. (F) Postoperative month six: fundus photo of submacular hemorrhage. (G) Postoperative month six: OCT of submacular hemorrhage through the macroaneurysm, which was the source of the original bleed.
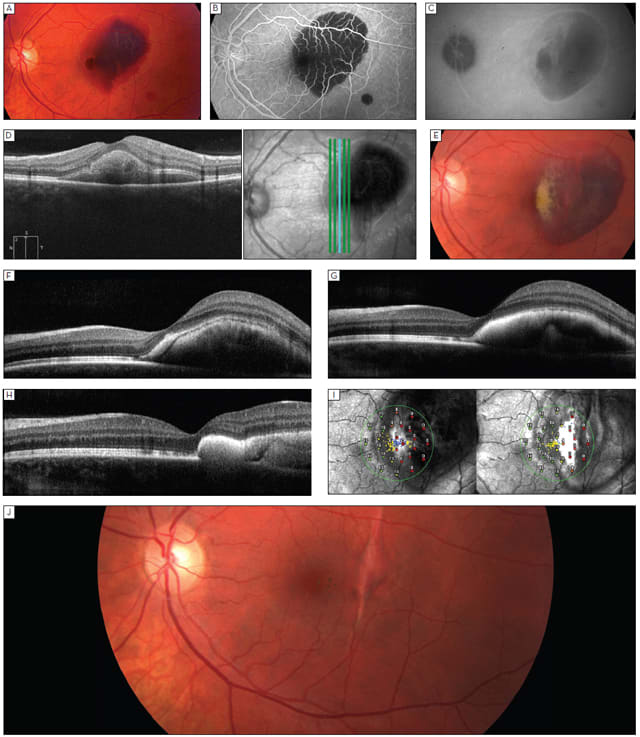
Figure 2. (A) Fundus photo of 28-year-old man with recent traumatic submacular hemorrhage. (B) FA of the same patient, confirming subfoveal location of blood. (C) Late ICG of recent traumatic submacular hemorrhage showing vertical linear streak suspicious for choroidal rupture. (D) Vertical OCT scan, showing subfoveal location of blood. (E) Fundus photo of a two-week-old traumatic submacular hemorrhage, showing evidence of clot dissolution. (F) Horizontal OCT scan through a two-week-old traumatic submacular hemorrhage following intravitreal TPA and C3F8 injection showing movement of blood temporally. (G) Horizontal OCT scan through a three-week-old traumatic submacular hemorrhage showing some reflectivity changes and flattening of the blood clot. (H) Horizontal OCT scan at week four showing temporal movement of the blood clot. (I) Serial OCT/SLO microperimetry studies at week two (left) and at week four (right). Note the improvement in central sensitivity along the edge of the resolving submacular hemorrhage and the improvement in stability of fixation. (J) Fundus photo of traumatic submacular hemorrhage at nine weeks showing resolution of blood and linear choroidal rupture scar.
The technique of PPV, subretinal TPA injection and air-fluid exchange is not without potential pitfalls. In addition to the inherent risk of retinal detachment with PPV, subretinal injection of TPA has been associated with a 27% risk of recurrent hemorrhage.1 There is some risk of RPE rip following subretinal TPA injection in cases with associated RPE detachments.1,6 There is also evidence from animal studies suggesting that the arginine carrier used in the formulation of TPA may have retinal and RPE toxicity at doses greater than 50 µm. Therefore, dosages limited to 50 µm or less are recommended.7
Given the surgical risks associated with PPV and subretinal TPA injection, a more conservative technique was developed by Wilson Herriot in 1997 for hemorrhagic events related to exudative AMD.2 The technique involved intravitreal pneumatic displacement using expansile gas such as perfluoropropane (C3F8) or sulfur hexafluoride (SF6) injected intravitreally with a 30-gauge needle. This was performed in conjunction with an intravitreal injection of TPA at a dose of 25 µg/0.1 mL, designed to avoid potential toxicity. Following the two injections, patients were instructed to assume a face-down position for at least 24 hours to allow the submacular hemorrhage to be displaced.
Introduction of this gas/TPA technique led to questions as to the efficacy of intravitreal vs subretinal TPA based on whether TPA had the ability to penetrate the retina. Arguments supporting intravitreal efficacy cited the presence of discontinuities in the ILM due to vitreous hemorrhage or other naturally occurring discontinuities of the ILM at the fovea, which helped provided access to the subretinal space from the vitreous cavity.2 However, in a direct comparison of subretinal vs intravitreal TPA in patients undergoing pneumatic displacement, better outcomes were achieved in the subretinal TPA injection group.1 Fifty-five percent of patients who received subretinal TPA and 22% of patients who received intravitreal TPA had complete displacement of the hemorrhage from the fovea.1
Even with successful displacement of the submacular hemorrhage, deterioration of visual function can progress due to any underlying disease.6 Adding intravitreal bevacizumab to intravitreal TPA and gas in cases of AMD was shown to improve visual outcomes.6,8,9 PPV combined with submacular injection of 10-20 µg of TPA in 0.05 mL of balanced salt solution (BSS) plus 1.25 mg bevacizumab in 0.05 mL BSS followed by air-fluid gas exchange using 20% SF6 gas has also been shown to be beneficial in treating subretinal hemorrhages in AMD patients.6 Similar results were obtained using intravitreal injections of 50 µg of TPA in 0.05 mL, with 0.3-0.4 mL SF6, followed by 1.25 mg bevacizumab in 0.05 mL BSS.9
To avoid the use of large peripheral retinectomies in cases of bullous hemorrhagic retina detachments, TPA has also been injected intravitreally 12-24 hours preoperatively, followed by PPV, peripheral retinotomy, drainage of the subretinal hemorrhage using perfluorocarbon liquid, and gas tamponade.10
No consensus exists as to the single best approach to use in cases of submacular hemorrhage. Intravitreal injection of gas ± TPA, PPV with subretinal or intravitreal TPA with air or expansile gas, and PPV with TPA and subretinal or intravitreal bevacizumab have each been shown to be successful in displacing the subretinal hemorrhage and improving visual acuity.6 Which approach to choose depends on an individual patient's visual acuity, his or her ability to comply with positioning, the extent of hemorrhage, and whether the depth of the hemorrhage is subretinal, sub-RPE or a combination of the two. Location of the hemorrhage in relation to the central macula is also an important consideration. Vision can be adversely affected if a hemorrhage superior to the fovea is pneumatically displaced into the fovea.3 When choosing the best approach for a particular case, the risks of rebleeding, vitreous hemorrhage and retinal detachment should all be considered and discussed with the patient. While each option is not without risk, each offers some opportunity for significant visual improvement. Left to resolve without treatment, submacular hemorrhages will most likely result in significant permanent visual loss. RP
REFERENCES
1. Hillenkamp J, Surguch V, Framme C, Gabel VP, Sachs HG. Management of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol. 2010;248:5-11.
2. Borrillo JL, Regillo CD. Treatment of subretinal hemorrhages with tissue plasminogen activator. Curr Opin Ophthalmol. 2001;12:207-211.
3. Sandhu SS, Manvikar S, Steel DH. Displacement of submacular hemorrhage associated with age-related macular degeneration using vitrectomy and submacular tPA injection followed by intravitreal ranibizumab. Clin Ophthalmol 2010;4:637-642.
4. Holland D, Wiechens B. Intravitreal r-TPA and gas injection in traumatic submacular hemorrhage. Ophthalmologica. 2004;218:64-69.
5. Sampangi R, Chandrakumar HV, Somashekar SE, Joshi GR, Ganesh S. SD-OCT to differentiate traumatic submacular hemorrhage types using automatic three-dimensional segmentation analysis. Ophthalmic Surg Lasers Imaging. 2011;42 Online:e32-36.
6. Treumer F, Klatt C, Roider J, Hillenkamp J. Subretinal coapplication of recombinant tissue plasminogen activator and bevacizumab for neovascular age-related macular degeneration with submacular haemorrhage. Br J Ophthalmol. 2010;94:48-53.
7. Singh RP, Patel C, Sears JE. Management of subretinal macular haemorrhage by direct administration of tissue plasminogen activator. Br J Ophthalmol. 2006;90:429-431.
8. Guthoff R, Guthoff T, Meigen T, Goebel W. Intravitreous injection of bevacizumab, tissue plasminogen activator, and gas in the treatment of submacular hemorrhage in age-related macular degeneration. Retina. 2011;31:36-40.
9. Meyer CH, Scholl HP, Eter N, Helb HM, Holz FG. Combined treatment of acute subretinal haemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: a retrospective pilot study. Acta Ophthalmol. 2008;86:490-494.
10. Oshima Y, Ohji M, Tano Y. Pars plana vitrectomy with peripheral retinotomy after injection of preoperative intravitreal tissue plasminogen activator: a modified procedure to drain massive subretinal haemorrhage. Br J Ophthalmol. 2007;91:193-198.
| Robert J. Lowe, MD, is a retinal fellow and Richard B. Rosen, MD, and Ronald C. Gentile, MD, are on the faculty of the New York Eye and Ear Infirmary. Dr. Lowe reports no financial interest in any product mentioned in this article. Dr. Rosen and Dr. Gentile report moderate financial interest in Genentech. Dr. Rosen can be reached via e-mail at rrosen@NYEE.EDU. |








