Simplified Repair of Simple to Complex Retinal Detachments
Vitrectomy without scleral buckling for rhegmatogenous retinal detachment.
Steve Charles, MD
Vitrectomy without scleral buckling for rhegmatogenous retinal detachment is often referred to as “primary vitrectomy,” a term that, at least initially, implied that scleral buckling was the standard of care and should be tried before resorting to vitrectomy. This “rescue therapy” approach is considered to be “conservative” and is applied in many specialties and disease processes, but it can produce the unintended consequence of delaying adoption of improved therapies and putting the patient through an unnecessary procedure.
Many prominent vitreoretinal surgeons refer to combined vitrectomy and scleral buckle, vit-buckle, as the “gold standard”—a ridiculous notion when applied to surgical therapy. Some surgeons use vitrectomy only for pseudophakic eyes, believing incorrectly that vitrectomy causes de novo nuclear sclerosis, when, in fact, it only causes progression of pre-existing nuclear sclerosis.
COMPLICATIONS OF SCLERAL BUCKLING
Patients spend substantial sums of money in the pursuit of emmetropia; LASIK, photorefractive keratotomy and refractive lens exchange have raised patients' expectations of life without glasses or contacts. Cataract surgery patients expect emmetropia; substantial research, product development and marketing efforts have been applied to toric intraocular lenses, multifocal IOLs and accommodative IOLs. Cataract surgeons have applied substantial effort to reduce the refractive effects of cataract surgery incisions, developing femtosecond laser–assisted cataract surgery, microincisional techniques and limbal relaxing incisions to improve refractive outcomes. Encircling buckles produce approximately 2.75 D of myopia—a completely unacceptable and unnecessary refractive outcome.
Proponents of scleral buckling often minimize the complications of the procedure. Many experienced scleral buckle surgeons state that they “never” produce strabismus, yet a high-quality prospective trial reported by the late Ron Michels, an excellent surgeon, demonstrated a significant incidence of increased tropias and phorias. Encircling buckles may result in damage to the superior oblique tendon, producing problematic vertical strabismus.
Fortunately, most buckle surgeons have given up the unnecessary practice of removing and reattaching extraocular muscles. Aggressive traction on retromuscle traction sutures, especially with small-diameter sutures, can “cheese-wire” intraocular muscle tendons and aggressive stripping of the intramuscular septum, Tenon's capsule and episclera, combined with cautery, can create adhesions, all of which contribute to post-operative strabismus.
More serious complications of scleral buckling include late intrusion of the buckle and buckle extrusion and infection. Intraoperative complications include a 5% incidence of retinal incarceration at the drainage site when using cut-down drainage, as well as bleeding related to the drainage site. Scleral, choroidal and retinal perforation with scleral sutures are not uncommon either, sometimes with serious consequences.
VIT-BUCKLES
Many vitreoretinal surgeons usually use encircling bands in conjunction with vitrectomy for repair of rhegmatogenous retinal detachment—so-called vit-buckles. I have not used this approach for two decades in order to eliminate buckling-induced refractive errors, strabismus, ptosis and pain, as well as to reduce operating times and therefore labor costs.
Brazitikos, Stangos and others have shown that vitrectomy without scleral buckling for retinal detachment produces better outcomes than vit-buckles. Patients would not want a vitreoretinal surgeon to use encircling bands when having vitrectomy for the repair of retinal detachment if they were informed about outcomes and complications.
VITRECTOMY SPECIFICS
Wide-angle visualization techniques and/or use of scleral depression are essential if vitrectomy is to be used for retinal detachment repair. Contact-based wide-angle visualization (Volk, AVI) produces a 10° greater field of view than noncontact-based visualization (BIOM, EIBOS) and eliminates all corneal asphericity (keratoconus, LRI, radial keratoplasty, penetrating keratoplasty, cataract surgery, LASIK, PRK). In addition, contact-based wide-angle visualization greatly reduces the need for ocular rotation to view the periphery, which reduces flexural forces on 25-gauge tools.
Just as with scleral buckling, all retinal breaks must be identified and treated with endophotocoagulation. Traction on the flap, as well as vitreous traction surrounding all breaks, must be eliminated to produce the achievable ~90% success rates. Internal drainage of subretinal fluid performed simultaneously with fluid-air exchange with a soft-tip cannula usually drains most of the subretinal fluid. If internal drainage is initiated prior to fluid-air exchange, posterior migration of subretinal fluid is reduced. Drainage retinotomy can be used if substantial posterior migration of subretinal fluid occurs or if the retinal breaks are very small and far peripheral, which can make internal drainage challenging.
Another option for the removal of subretinal fluid is perfluorocarbon liquids; N-perfluoro-octane (PFO) is the preferred agent because the interface is visible, unlike perflurodecalin. PFO will remove all subretinal fluid if the optimal techniques are used, while internal drainage of sub-retinal fluid plus fluid-air exchange always leaves a thin layer of fluid that must be pumped out by the RPE. Complete removal of subretinal fluid enabled by PFO may allow the use of a shorter-acting gas or even air for surface-tension management.
Because PFO causes subretinal fluid to float anteriorly, care must be taken to remove all subretinal fluid anterior to the retinal breaks to enable the surrounding of all breaks with endolaser. This can be done by extending the break to the ora or by making a very peripheral, small drainage retinotomy, but the best approach is to drain fluid slowly through the retinal break using a 25-g soft-tip cannula just when the PFO reaches the break. Care must be taken to not remove any PFO. The MedOne 25-g dual-bore VFI is ideal for injecting PFO, while allowing infusion fluid egress to maintain appropriate intraocular pressure.
25-GAUGE SUTURELESS VITRECTOMY
The author uses a 25-g sutureless approach for all vitrectomies, including rhegmatogenous retinal detachments, posterior vitreous retinopathy, giant breaks and diabetic traction retinal detachments. Just as today's patients expect emmetropia without strabismus or ptosis, as discussed above, they expect a painless procedure and a noninflamed eye. A noninflamed, pain-free eye is not achievable with 20-g sutured wounds or vit-buckles. Contrary to what some surgeons believe, 25-g vitrectomy fluidics are preferable to 23-g or 20-g fluidics for all RD cases, simple to complex, because port-based flow limiting due to a smaller lumen reduces pulsatile vitreoretinal traction.
The author strongly recommends use of the highest possible cutting rate for all tasks and all cases—especially for giant breaks and other retinal detachment cases. The author uses only the Alcon Constellation Vision System, with Ultravit cutter, currently at 5,000 cuts/minute. Sutured-on contact lenses damage the conjunctiva, cause sub-conjunctival bleeding and are inappropriate for sutureless, transconjunctival surgery.
Sutureless, transconjunctival, microincisional vitrectomy is ideal for retinal-detachment repair. In the author's opinion, vit-buckles are never indicated; the focus should be on microincisional vitrectomy techniques using wide-angle visualization to repair retinal detachments without causing pain, refractive error, strabismus, ptosis, cosmetic problems and longer, more costly operating times.
REOPERATION FOR RETINAL DETACHMENT OR EPIMACULAR MEMBRANE WITHOUT REMOVING SILICONE OIL
Many surgeons unnecessarily remove silicone oil when reoperating for epimacular membrane or recurrent retinal detachment. For over 25 years, the author has operated “under” silicone oil when reoperating these cases.
Key advantages to this approach are: decreased surgical trauma, especially to the lens and cornea; realistic assessment of traction removal; and greatly decreased operating times and, therefore, labor cost. Reduced damage to the conjunctiva, Tenon's capsule and episclera is especially important if the patient has glaucoma and may require filtration surgery or if a filtering bleb is present.
Silicone oil produces approximately 50% less interfacial surface tension than air or gas interface with infusion fluid or aqueous humor; therefore, reoperation with oil in place gives a realistic assessment of the relative force due to interfacial tension and retinal-surface contraction. Many surgeons have performed fluid-air exchange, endophotocoagulation, and air-silicone exchange for cases of proliferative vitreoretinopathy, only to find the retina was partially detached the next day because of the surface-tension disparity.
Typical operating times are 15 minutes or less for epimacular membrane cases and 30 minutes for PVR or diabetic traction retinal detachment reoperation. The time savings are significant because it is easier on the patient, and operating room costs, 70% of which are labor, are reduced.
The author has used two-port 25-g vitrectomy for re-operation in eyes with silicone oil for the last seven years. The 25-g, two-port method is performed using the Alcon Viscous Fluid Control Pak 25-g cannula to inject oil sequentially after internal drainage of subretinal fluid reduces the IOP. The two-port method is effective because the viscosity of the oil prevents reflux from the 25-g cannulas during instrument exchange.
Forceps membrane peeling using the Alcon 25-g DSP ILM forceps (Figure 1), scissors segmentation and scissors delamination using the Alcon 25-g DSP curved scissors, cutter delamination with the Ultravit at 500 cuts/minute, retinectomy, internal drainage of subretinal fluid, removal or resection of subretinal traction elements, retinectomy and endophotocoagulation all work well with silicone oil in place. It is necessary to keep the port of the cutter within the preretinal fluid layer and subretinal space and to add silicone oil when the IOP decreases. A vacuum setting of 600-650 mm Hg is required for cutter removal of residual vitreous, retinectomy or cutter delamination. A maximum proportional vacuum setting of 600-650 mm Hg is required for internal drainage of subretinal fluid using a 25-g soft-tip cannula. Extreme care should be taken to keep silicone oil out of the soft-tip cannula to prevent plugging. PVR and diabetic traction retinal cases often require retinectomy; this is accomplished by removing contracted, stiff retina, epiretinal membrane and subretinal fluid or subretinal oil together using the cutter. Large vessels should be coagulated first with bipolar diathermy with the tip in contact with the vessel because, unlike infusion fluid, silicone oil is not conductive.

Figure 1. Inside-out forceps membrane peeling, with Alcon 25-gauge DSP ILM forceps, is used to remove ERMs. Pics, viscodissection and forceps with one blade under the ERM are more likely to damage the retinal surface.
MEDIUM-TERM PFO FOR REPAIR OF INFERIOR RETINAL DETACHMENTS
The author has for approximately 15 years used medium-term N-perfluorooctane for the repair of inferior rhegmatogenous retinal detachments; inferior PVR; and inferior, temporal and nasal giant breaks. Use of the medium-term PFO technique for inferior giant breaks eliminates the slippage problem; positioning the patient on his or her side allows use of this technique for temporal and nasal giant breaks as well.
Either PFO-gas or PFO-air-oil exchange is required for superior giant breaks. The medium-term PFO technique eliminates the need for face-down positioning, as well as scleral buckles, and it facilitates driving, standing, sitting and flying. The only negatives are: (1) a brief 25-g procedure is required two weeks later to remove the PFO and (2) residual droplets are common.
Liquid perfluorocarbons (heavy liquids) were developed by Stanley Chang for unfolding giant breaks and stabilizing the retina during membrane peeling in PVR cases (Figure 2). Although FDA approval of PFO was for intraoperative use, there was no sound scientific evidence to suggest that toxicity would occur if the agent were left in place for two weeks to allow for maximum tensile strength of laser retinopexy.

Figure 2. The AVC and PVC undergo hypocellular contraction of the collagen fibers, resulting in confluence and an FP sheet. The FP component should be removed before forceps membrane peeling of star folds.
The author's technique uses 25-g vitrectomy with the Alcon Constellation Vision System with an Ultravit cutter at 5,000 cuts/minute. It is essential to remove traction on flaps or flap tears specifically, as well as vitreous surrounding all retinal breaks (Figure 3). The PFO is then injected with the Alcon VFC at 10 psi using the MedOne Dual Bore 25-g cannula to allow egress of infusion fluid for IOP stabilization (Figure 4). The injection should be initiated nasal to the optic nerve, away from any retinal breaks (Figure 5). If subretinal fluid does not reflux spontaneously from retinal breaks, the soft-tip cannula should be placed through a retinal break into the subretinal space to initiate drainage of highly viscous fluid.

Figure 3. Medium-term PFO requires compulsive, 360° removal of vitreous traction using wide-angle visualization.
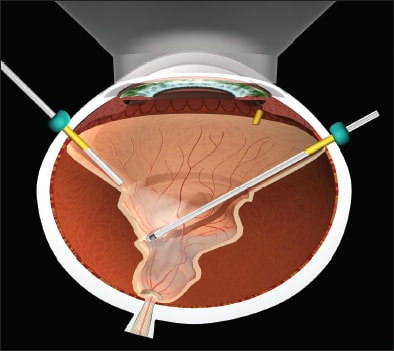
Figure 4. Following FAX and IDS and usually retinopexy, a silicone injector, such as the Alcon VFC, is used to inject silicone oil through a short, thin-wall cannula, while air through the infusion cannula maintains IOP, and air egress occurs with an extrusion cannula behind the lens/IOL or in the anterior chamber of aphakic eyes. The injection is stopped and the infusion line clamped with a hemostat when silicone enters the infusion cannula.

Figure 5. PFO injection over the optic nerve with a 25-gauge dual-bore cannula. ALL IMAGES ORIGINALLY APPEARED IN: CHARLES S, CALZADA J, WOOD B. VITREOUS MICROSURGERY. 5TH ED. PHILADELPHIA, PA; LIPPINCOTT, WILLIAMS & WILKINS; 2011. IMAGES APPEAR COURTESY OF WOLTERS KLUWER HEALTH.
Subretinal fluid displaced anterior to retinal breaks should be removed by extending the soft-tip cannula under the retina anteriorly or by making a very small, very anterior drainage retinotomy. After complete retinal reattachment, confluent (painting) endolaser is placed around all retinal breaks, lattice degeneration and thin or suspicious areas. A full fill of PFO is essential to reduce break-up into multiple, small bubbles. The Alcon valved cannulas prevent loss of PFO during tool exchange, as well as cannula removal at the end of the case.
Some patients develop a mild foreign body reaction with deposits on the posterior surface of the lens or IOL and along the retinal vessels. This reaction will clear after the PFO is removed, but the problem can be reduced by treating with topical difluprednate four times per day for two weeks. Initially, the author used intravitreal triamcinolone, but sustained-release triamcinolone may accumulate on the lens or IOL surface and not clear for months.
The PFO is removed with 25-g sutureless vitrectomy technique and a soft-tip cannula. A 30-g cannula through the limbus attached to linear extrusion is used to remove PFO droplets that inevitably find their way into the anterior chamber in phakic and pseudophakic eyes. Multiple fluid-air exchanges can be effective at reducing the problem of residual droplets. Droplets in the anterior chamber can be easily removed at the slit lamp under topical anesthesia with a lid speculum in place, by placing a 30-g needle through the limbus, bevel up at the 6 o'clock position.
Perfluoro-octane is almost twice the density of aqueous and two-thirds the viscosity, so no vacuum is required; a TB syringe with the plunger removed is used to position the needle. This technique must be repeated several times as the chamber shallows and refills or on multiple office visits. Removal of residual droplets is necessary to prevent glaucoma.
SUMMARY
Several methods have been described that reduce surgical trauma, complications and operating time while not compromising attachment or visual outcomes. It is important for every vitreoretinal surgeon to update techniques and technologies constantly and not to fall into the trap of the status quo, ie, the “gold standard.” RP
| Steve Charles, MD, is clinical professor of ophthalmology at the University of Tennessee College of Medicine in Memphis. He reports significant financial interest in Alcon. Dr. Charles can be reached at scharles@att.net. |








