Diabetic Retinopathy: The Masqueraders
Clinical hallmarks of DR can be associated with a variety of diseases.
IRENE A. BARBAZETTO, MD
Diagnosing diabetic retinopathy (DR) is considered part of the daily "bread and butter" of the retinal specialty. We are familiar with the hallmarks of the retinal manifestations of the disease, which include but are not limited to intraretinal hemorrhages, microaneurysms, capillary dropout, telangiectasia, cotton wool spots and exudates, as well as macular edema, retinal neovascularization, vitreous hemorrhages and tractional retinal detachments in the advanced stages.
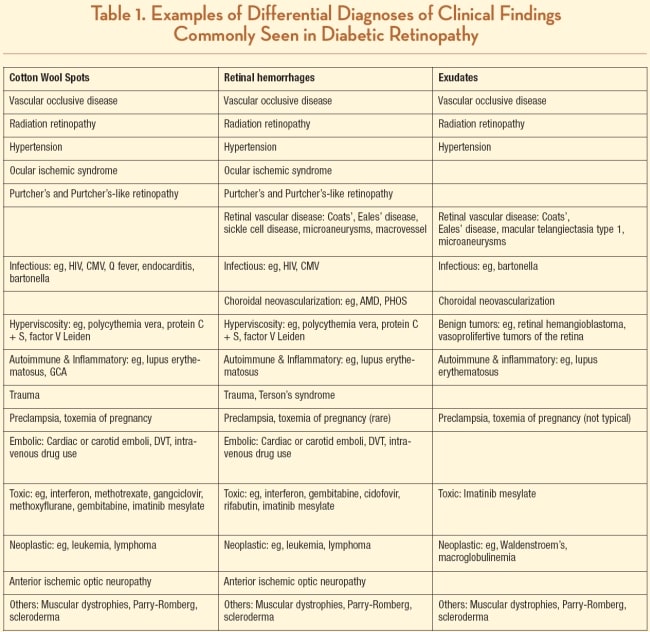
At times, however, patients present with retinal pathology mimicking DR, and it is necessary to consider other etiologies in the differential diagnosis, especially in the presence of a negative work-up for diabetes (see Table 1). These conditions present with features typically seen in DR, either individually or in combination. While the most common differential diagnoses include hypertensive retinopathy, radiation retinopathy, ocular ischemic syndrome and vascular occlusive disease, other less frequent conditions — such as infectious etiologies, inflammatory and autoimmune diseases, cancer and paraneoplastic retinopathies, muscular dystrophies and craniofacial disorders, among others — need to be considered.
CANCER AND PARANEOPLASTIC RETINOPATHIES
In most cancer patients, the diagnosis has been established before presenting to an ophthalmologist. However, in rare instances, ocular symptoms and examination lead to the diagnosis of leukemia or other blood dyscrasias. While many of the lymphomas and leukemias can directly infiltrate the retina and choroid with tumor cells, it is usually the associated retinopathy, secondary to hyperviscosity, anemia or thrombocytopenia, that closely resembles diabetic retinopathy.
Leukemia is known to present with direct leukemic infiltration of the retina or leukemic retinopathy, characterized by retinal hemorrhages (intraretinal, subretinal or subhyaloid), cotton wool spots and vascular tortuosity. The latter posterior manifestations seem to occur more frequently in adult patients with myeloid leukemia.1
Mandava and coworkers described an impressive case of a patient with chronic myelogenous leukemia presenting with severe retinal fibrovascular proliferation, tractional retinal detachment and vitreous hemorrhages.2 Other cases showed proliferative disease,3 peripheral nonperfusion4 and, again, retinal hemorrhages.5 Optic nerve infiltrations similar to diabetic papillopathy have been described as well.6 Rarely, serous retinal detachments as part of tumor recurrence in acute myelogenous leukemia have been reported.7
Lymphoma in general is considered to be one of the "Great Masqueraders." It can involve almost all ocular structures, including the retina, choroid, vitreous and optic nerve. While most changes are infiltrative in nature, retinal hemorrhages have been described in patients with T-cell lymphoma,8 and optic nerve infiltration may resemble diabetic papillopathy.9
Waldenström's macroglobulinemia is a rare, indolent (ie, slow-growing) non-Hodgkin lymphoma. The microangiopathy seen in some patients is thought to be the direct result of hyperviscosity and increased arteriovenous passage time.10 Retinal changes may include microaneurysms, hemorrhages and lipid exudation. Exudative macular detachments described in Waldenström's macroglobulinemia usually lack the pronounced cystic changes seen in diabetic macular edema.11
Multiple myeloma is a malignancy of plasma cells in the bone marrow mainly characterized by the appearance of paraproteins (Bence-Jones) in serum and urine. Advanced disease can lead to so-called endorgan damage, including hypercalcemia, renal insufficiency, anemia and bone lesions. Findings in the retinal vasculature that have been described include tortuosity, scattered hemorrhages, microaneurysms throughout the posterior pole, and, in the later stages, retinal venous occlusive disease.12

Radiation maculopathy from iodine 125–treated peripapillary choroidal melanoma. Retinal hemorrhages, microaneurysms, exudates and cotton wool spots are present; these are all often symptoms of diabetic retinopathy.
HYPERVISCOSITY SYNDROMES
Hyperviscosity is thought to be the origin of microangiopathy involving the retinal vasculature in many of these cases. Polycythemia vera is an example of a chronic myeloproliferative disease with hyperviscosity secondary to an overproduction of erythrocytes and platelets. Ocular findings include retinal vascular occlusions, hemorrhages, cotton wool spots, ischemic optic neuropathy, vascular tortuosity and markedly delayed arterial and venous recirculation time on fluorescein angiography.13-15
Other conditions resulting in hypercoagulable states, such as Protein C and S deficiencies, factor V Leiden, antithrombin III deficiency, essential thrombocythemia, and disseminated intravascular coagulopathy, may also present with retinal hemorrhages, cotton-wool spots and vascular occlusions.11
OCULAR ISCHEMIC SYNDROME
Ocular ischemic syndrome is a condition that can be either unilateral or bilateral in nature and is characterized by delayed choroidal filling time on fluorescein angiography, possible disc and retinal vessel staining, peripheral ischemia, microaneurysms, macular edema, cotton-wool spots and even neovascularization. The lack of vascular tortuosity in the presence of narrow arteries and dilated retinal veins may give retinal specialists a clue toward the correct diagnosis; so does the presence of anterior-segment inflammation.
Unlike diabetes, these patients can first present with ocular pain, in addition to sudden or gradual loss of vision. Carotid artery stenosis (internal and external), giant cell arteritis and vascular occlusive disease of the aortic arch, ophthalmic artery, central retinal artery and vein, as well as cilliary arteries, have to be ruled out.16-20
INFLAMMATORY/AUTOIMMUNE DISORDERS
Retinal findings have been described in several inflammatory, collagen-vascular and autoimmune disorders, such as systemic lupus erythematosus, panarteritis nodosum, sarcoidosis and Takayasu arteritis. Cotton-wool spots and, less frequently, hemorrhages seem to be the most common findings, except for Takayasu arteritis, which presents as an ocular ischemic syndrome with widespread microaneurysms.21
Systemic lupus erythematosus (SLE). The most common retinal findings with SLE are cotton-wool spots. Retinal hemorrhages, ischemia, microaneurysms, telangiectasia and neovascularization are less frequently observed.11 Rare cases can show vascular occlusive disease and choroidal neovascularizations.
MUSCULAR DYSTROPHIES
Facioscapulohumeral muscular dystrophy (FSHD), also known as Landouzy-Dejerine, is an autosomal-dominant inherited form of muscular dystrophy affecting the skeletal muscles of the face, shoulder and upper arms. Sensorineural hearing loss, retinal telangiectasia, exudates and retinal ischemia have also been described.22 Most cases of FSHD are associated with the deletion of integral copies of a tandem repeated 3.2kb unit (D4Z4 repeat) at the subtelomeric region 4q35. Genetic testing is available for patients.23
Duchenne's muscular dystrophy is a severe and rapidly progressing form of muscular dystrophy with X-linked inheritance (dystrophin gene Xp21). It usually becomes symptomatic in early childhood and initially affects lower extremities and the pelvis. Only a few case reports of retinal involvement are available. One of the most striking cases has been reported by Ober and coauthors; this 18-year-old patient presented with widespread vascular abnormalities, including beading, tortuosity, neovascularization and leakage.24
CRANIOFACIAL ABNORMALITIES
Craniofacial abnormalities can affect the eye in many ways, most frequently due to involvement of the orbital configuration and muscular alignment. Rarely, retinal disorders are described.
Parry-Romberg is characterized by slowly progressive hemifacial atrophy starting in the first or second decade of life. Several neurological symptoms (including seizures, intracranial vascular malformations and trigeminal neuralgia) have been described, in addition to other symptoms, such as alopecia and vitiligo. The origin is unknown and several hypotheses with regard to a potential infections/inflammatory etiology vs a disruption or disturbance of the sympathetic innervations have been proposed. Ophthalmic findings include a wide variety of problems mostly related to the progressive atrophy of periorbital and ocular tissues, eg, ocular misalignment, enophthalmus and pupillary abnormalities. In the posterior segment, retinal neovascularization, telangiectasia, peripheral ischemia retinal vasculitis and exudative detachments have been reported.11,25-27
Linear scleroderma "en coup de sabre" is a localized form of scleroderma with facial involvement but lack of systemic fibrosis. Together with Parry-Romberg, it is considered to be part of the linear scleroderma spectrum. In the retinal vasculature, tortuosity, neovascularization, ischemia, cotton wool spots, telangiectasia and lipid exudation have been described.11,28
OTHER VASCULAR ABNORMALITIES
Macular telangiectasia type 1 has many overlapping features with Coats' disease. Both can present with diabetic features, including microaneurysms, exudates, hemorrhages and telangiectasia. Frequently, the age of the patient can aid in the correct diagnosis. But delayed diagnosis or adult-onset Coats' disease represents greater diagnostic challenges. Less well known is that macular telangiectasia type 2 may also show parafoveal microaneurysms, in addition to the typical telangiectatic changes on fluorescein angiography.11
Retinal macrovessels are a rare congenital disorder with a large "aberrant" vessel crossing the horizontal raphae. Adjacent vascular changes have been described, which can include vascular tortuosities, arteriovenous anastomosis, changes in the macular capillary bed, aneurysms and hemorrhages.29-31
CONCLUSION
In summary, clinical hallmarks of diabetic retinopathy can be associated with a wide variety of diseases. After excluding the most common differential diagnoses, rare conditions have to be considered. While this article gives an overview of several diseases, there are obviously many more conditions that directly or indirectly can resemble features of diabetic retinopathy.
It is important to remember that the ophthalmologist may aid in making the correct diagnosis and facilitating these patients' access to appropriate care and treatment. RP
REFERENCES
1. Wu L. Leukemias. eMedicine from WebMD Web site. http://emedicine.medscape.com/article/1201870-overview. Accessed June 30, 2010.
2. Mandava N, Costakos D, Bartlett HM. Chronic myelogenous leukemia manifested as bilateral proliferative retinopathy. Arch Ophthalmol. 2005;123:576-577.
3. Hoerauf H, Bopp S, Laqua H. [Proliferative retinopathy in chronic myeloid leukemia]. Klin Monbl Augenheilkd. 1994;205:226-230.
4. Nobacht S, Vandoninck KF, Deutman AF, Klevering BJ. Peripheral retinal nonperfusion associated with chronic myeloid leukemia. Am J Ophthalmol. 2003;135:404-406.
5. Dhaliwal RS, Schachat AP. Leukemias and lymphomas. In: Ryan SJ, ed. Retina. 3rd ed. Vol. 2. Mosby; St. Louis, MO; 2001.
6. Camera A, Piccirillo G, Cennamo G, et al. Optic nerve involvement in acute lymphoblastic leukemia. Leuk Lymphoma.1993;11:153-155.
7. Wu L, Calderon M, Hernandez G, et al. Bilateral exudative retinal detachment as the first sign of relapsing acute myelogenous leukaemia. Clin Experiment Ophthalmol. 2006;34:623-625.
8. Edwards J, Simmons E, Cordero S, et al. Retinal hemorrhages as a presenting sign in an adolescent patient with hepatosplenic gamma-delta T-cell lymphoma. Pediatr Blood Cancer. 55:190-102.
9. Dayan MR, Elston JS, McDonald B. Bilateral lymphomatous optic neuropathy diagnosed on optic nerve biopsy. Arch Ophthalmol. 2000;118:1455-1457.
10. Dobberstein H, Solbach U, Weinberger A, Wolf S. Correlation between retinal microcirculation and blood viscosity in patients with hyperviscosity syndrome. Clin Hemorheol Microcirc. 1999;20:31-35.
11. Yannuzzi LA. The Retina Atlas. Elsevier; Philadelphia, PA; 2010.
12. Orellana J, Friedman AH. Ocular manifestations of multiple myeloma, Waldenstrom's macroglobulinemia and benign monoclonal gammopathy. Surv Ophthalmol. 1981;26:157-169.
13. Ahn BY, Choi KD, Choi YJ, et al. Isolated monocular visual loss as an initial manifestation of polycythemia vera. J Neurol Sci. 2007;258:151-153.
14. Parija S, Mohapatra MM, Pattnaik BK. Polycythemia vera presenting with bilateral papilledema: a rare case report. Indian J Ophthalmol. 2008;56:327-329.
15. Tonz MS, Rigamonti V, Iliev ME. Simultaneous, bilateral anterior ischemic optic neuropathy (AION) in polycythemia vera: a case report. Klin Monbl Augenheilkd. 2008;225:504-506.
16. Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int Ophthalmol. 1988;11:239-251.
17. Chen CS, Miller NR. Ocular ischemic syndrome: review of clinical presentations, etiology, investigation, and management. Compr Ophthalmol Update. 2007;8:17-28.
18. Casson RJ, Fleming FK, Shaikh A, James B. Bilateral ocular ischemic syndrome secondary to giant cell arteritis. Arch Ophthalmol. 2001;119:306-307.
19. Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. 1997;104:859-864.
20. Chuah JL, Ghosh YK, Richards D, Shun-Shin GA. Ocular ischaemic syndrome: a medical emergency. Lancet. 2006;367:1370.
21. Karam EZ, Muci-Mendoza R, Hedges TR, 3rd. Retinal findings in Takayasu's arteritis. Acta Ophthalmol Scand. 1999;77:209-213.
22. Fitzsimons RB, Gurwin EB, Bird AC. Retinal vascular abnormalities in facioscapulohumeral muscular dystrophy. A general association with genetic and therapeutic implications. Brain. 1987;110:631-648.
23. Wijmenga C, Padberg GW, Moerer P, et al. Mapping of facioscapulohumeral muscular dystrophy gene to chromosome 4q35-qter by multipoint linkage analysis and in situ hybridization. Genomics. 1991;9:570-575.
24. Ober MD, Del Priore LV, Tsai J, et al. Diagnostic and therapeutic challenges. Retina. 2006;26:426-67.
25. Bellusci C, Liguori R, Pazzaglia A, et al. Bilateral Parry-Romberg syndrome associated with retinal vasculitis. Eur J Ophthalmol. 2003;13:803-806.
26. Gass JD. Parry-Romberg syndrome. Arch Ophthalmol. 1999;117:1099.
27. Bandello F, Rosa N, Ghisolfi F, Sebastiani A. New findings in the Parry-Romberg syndrome: a case report. Eur J Ophthalmol. 2002;12:556-558.
28. Minasian M, Stanford M, Graham E, Denton CP, Black C. Bilateral ischaemic retinal vasculopathy in scleroderma. Br J Ophthalmol. 2005;89:1064-1065.
29. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16:74-75.
30. Koizumi H, Iida T, Mori T, et al. Retinal arteriolar macroaneurysm and congenital retinal macrovessel. Ophthalmic Surg Lasers Imaging. 2009;40:513-515.
31. de Crecchio G, Mastursi B, Alfieri MC, Pignalosa B. Congenital retinal macrovessel. Ophthalmologica. 1986;193:143-145.
| Irene A. Barbazetto, MD, is a retina fellow at the Harkness Eye Institute of Columbia University and practices with Vitreous-Retina-Macula Consultants in New York. She reports no financial interest in any products mentioned in this article. Dr. Barbazetto can be reached via e-mail at ibarbazetto@gmail.com. |








