Ready to Make the Switch?
How to take advantage of spectral domain OCT in a practical and productive manner.

By Srinivas Sadda, MD, and Alexander Walsh, MD
There is no question that spectral domain optical coherence tomography (SD-OCT), not its time domain predecessor, is the retinal imaging technology for the immediate future. The questions for individual practices have therefore become when to make the switch and which of the available instruments to choose.
For our practices at the University of Southern California's Doheny Eye Institute, we have asked and answered these key questions. Here, we explain why we adopted SD-OCT quickly and the criteria we used for comparing instruments. One SD-OCT device that stands out, not only for its excellent image quality and performance, but also for real-world clinical practice efficiency, is the Topcon 3D OCT-2000.
WHY IS SPECTRAL DOMAIN SUPERIOR?
In general, SD-OCT technology is an improvement over time domain technology in several important ways. Its super-luminescent diode light source uses a wider bandwidth, which provides higher axial resolution (eg, 5-7 μm vs. 8-10 μm for Stratus OCT [Carl Zeiss Meditec]). Higher axial resolution contributes to better visualization of very fine retinal structures, such as the photoreceptor inner segment (IS)/outer segment (OS) junction.
An even bigger advantage of SD-OCT, however, is its faster data acquisition speed. Using a spectrometer and mathematics (Fourier transform) instead of a moving reference mirror, the new SD-OCT systems can place 20,000-40,000 A-scans per second, which far exceeds the 400 A-scans per second capability of time domain. At these higher speeds, multiple B-scans can be averaged to better suppress noise. Furthermore, the retina is mapped much more densely and completely. The dense pattern of A-scans allows the construction of 3-D illustrations of the retina (Figure 1), and in contrast to time domain OCT, data between scan lines is not skipped or missed and need not be interpolated.

Figure 1. The dense pattern of A-scans acquired by spectral domain OCT allows the construction of 3-D illustrations of the retina.
Speed brings other benefits as well. It makes SD-OCT images less susceptible to motion artifact related to patient eye movement. The large amount of data acquired contains invariant landmarks, such as blood vessels, that can be aligned with fundus images to allow for more precise comparisons of OCT data from one patient visit to the next (Figures 2 and 3).
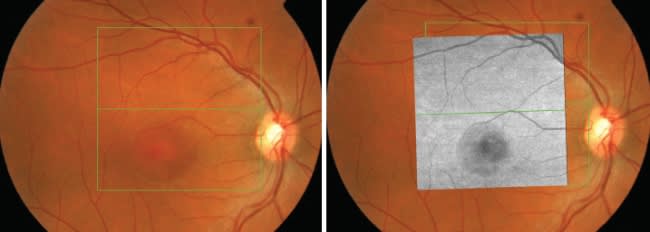
Figures 2 and 3. One benefit derived from the data acquisition speed of spectral domain OCT is the ability to register scans with a fundus image, which improves the precision of patient monitoring from visit to visit.
SD-OCT ENHANCES PATIENT CARE
While the improved technical capabilities of SD-OCT are intriguing, it is the technology for the future because it can enhance patient care. Based on the results of studies we have conducted, SD-OCT is more sensitive than time domain OCT, and the sensitivity adds valuable information to the patient management decision-making process.
In one of our studies,1 we prospectively obtained SD-OCT scans with the Topcon 3D OCT of 50 eyes of patients who were undergoing conventional time domain imaging. Two masked physician graders evaluated each set of data for the presence of various clinical findings. Findings from all gradings were combined to form the ground truth findings for each case. We found the overall sensitivity of SD-OCT to be 97% compared with 83% for time domain OCT. SD-OCT was more sensitive in the detection of several findings, including epiretinal membrane (96% vs. 83%), subretinal fluid (SRF) (100% vs. 86%), diffuse intraretinal edema (94% vs. 80%) and the hyaloid face (98% vs. 88%).
In another of our studies, for which results have not yet been published, we retrospectively reviewed the datasets from 62 eyes of 45 patients receiving treatment with ranibizumab (Lucentis, Genentech) or bevacizumab (Avastin, Genentech) who had simultaneous time domain and SD-OCT imaging within 1 week of injection. In these patients, SD-OCT detected at least 1 feature [cystoid macular edema (CME), SRF, and/or pigment epithelial detachment (PED)] that time domain did not in 12 of 62 cases (19%). SD-OCT revealed at least 1 larger feature (CME, SRF, PED) in 38 of 62 cases (61%), while time domain revealed 1 larger feature in only 1 of 62 cases (2%). Finally, if the presence of CME, SRF or PED were used as the only indication for retreatment, SD-OCT would have changed the management decision made in 6 of the 62 cases (10%).
Studies such as these and our experience imaging more than 1000 patients with both time domain and SD-OCT have convinced us that SD-OCT is the new standard of care. We no longer feel comfortable treating patients without the additional information it provides. We expect the technology to continue to impact many areas, including vitreoretinal interface disease, glaucoma, management of neovascular agerelated macular degeneration (AMD) (Figures 4 and 5), explaining the cause of vision loss (Figure 6), and (with advances in software) measuring the effects of therapy.
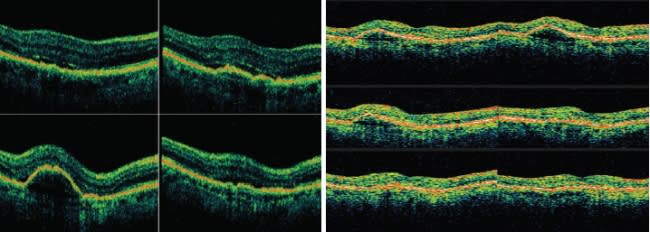
Figures 4 and 5. Spectral domain OCT (left) revealed persistent subretinal fluid in this patient undergoing treatment with ranibizumab (Lucentis). Fluid was not easily detected with time domain OCT (right).
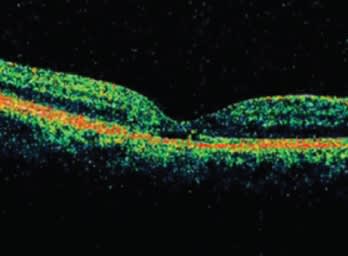
Figure 6. Spectral domain OCT helped to explain vision loss in this patient by detecting photoreceptor outer segment disruption. Time domain OCT findings were unremarkable.
CHOOSING AN INSTRUMENT
The available SD-OCT instruments share some attributes, such as the ability to create 3-D retinal representations, but differences between them exist as well. When deciding which instrument to purchase, a practice should consider features related to hardware, image quality and software.
HARDWARE
• With regard to scanning speed, keep in mind that higher speeds can often reduce native image quality thus requiring averaging of B-scans to obtain good quality imaging. B-scan averaging, however, proportionally reduces the effective A-scan acquisition speed. For example, even if an SD-OCT device scans at 40,000 A-scans/second, if 9 B-scans are averaged at each scan location, the effective scanning rate (with respect to scanning density) is reduced to only 4,444 A-scans/second.
• Some instruments utilize eye/fixation tracking technology, which can improve the averaging/overlapping of images and registration/alignment. Tracking can have drawbacks, such as slowing the acquisition of 3-D scans in patients with poor fixation and limiting the number of B-scans per 3-D scan. The latter can result in less dense sampling and an inability to acquire sufficient images in some patients.
• Ancillary imaging modalities combined with SD-OCT in one device can be helpful, but too many could tie up the instrument during busy clinic times.
• An SD-OCT instrument should be easy to use and patient-friendly to ensure efficient clinical operation.
• The manufacturer should have a proven track record with high-quality products, service and support.
IMAGE QUALITY
• As explained previously in this article, SD-OCT is more sensitive than time domain OCT. However, this does not mean the vitreous should appear black. A useful rule of thumb is to look for a device that shows vitreous tissue interfaces as well as a certain amount of "white" noise in the vitreous cavity. This helps to ensure vitreomacular traction problems will be noticeable.
• It is also helpful to view the outer retinal signal in every B-scan. It should appear consistently bright, especially in patients with subretinal pathology. If intensities in the outer retina are too dark, it may be difficult to detect subretinal fluid and distinguish subtle findings from normal tissue.
• Request to see not only B-scans from normal eyes but also images from eyes with various retinal diseases.
• Because SD-OCT is more sensitive than time domain OCT, some instruments may be more sensitive to media opacities. Therefore, when evaluating instruments, review scans from patients with corneal disease and cataracts in addition to scans that show retinal disease.
• Examine B-scan images in pseudocolor. Grayscale images may hide substantial amounts of noise and can make an inferior instrument seem clinically acceptable.
• Variations in the pseudocolor display between B-scans from the same patient may indicate signal quality problems.
• Review all images from sample 3-D scans, not just line scans. High-resolution line scans may look terrific, but the lower resolution 3-D scans may be inadequate for clinical purposes.
SOFTWARE
• SD-OCT data should be able to align with other imaging modalities to allow precise intervisit comparisons of measurements and reciprocal point-and-click functionality (for example, click on a fundus image to see OCT data from that region, or click on a B-scan to see the corresponding point on the retina). Several factors affect this ability. Therefore, be sure to understand how each device detects invariant landmarks and uses them for registration.
Also, eye movements disturb the relationship between invariant landmarks within a given OCT scan. Longer acquisition times and poor patient fixation further compound this problem. Short acquisition times minimize the likelihood of large saccades during image capture but do not completely solve the problem, so it is important to understand how each SD-OCT device aligns its data in the presence of saccades and fixation losses.
• Flexible image manipulation options are a useful feature to have with an SD-OCT instrument. C-scan sectioning and the ability to spin, roll or slice 3-D illustrations of retinal tissue in a realistic manner are available on many devices. Custom slicing, such as circular or oblique sectioning, is also possible.
• Many OCT users have accumulated large banks of time domain data that they will want to access in the future. For purposes of simplicity and efficiency, well-designed SD-OCT devices should include the ability to import time domain data to the new instrument's database, so a single device can be used for OCT data storage and comparisons.
• SD-OCT can generate hundreds of B-scans compared with the 6 radial B-scans typically provided by time domain systems. It is not advisable, or even feasible, to print all of them in color for individual viewing. Therefore, software for viewing and analyzing data that is easy to use for doctors and clinic staff members is a must.
• The best software systems will include networking support for the transfer of large OCT files rapidly and directly to patient rooms.
• Existing time domain OCT instruments are known to have problems with retinal boundary identification and layer segmentation, especially in the presence of AMD. In today's era of anti-vascular endothelial growth factor therapy, which is guided by subretinal findings such as fluid and PED, it is increasingly important for SD-OCT software to correctly identify tissue boundaries and extract clinically relevant measurements from images.
• Multiethnic normative databases, including optic nerve and macula, are also desirable.
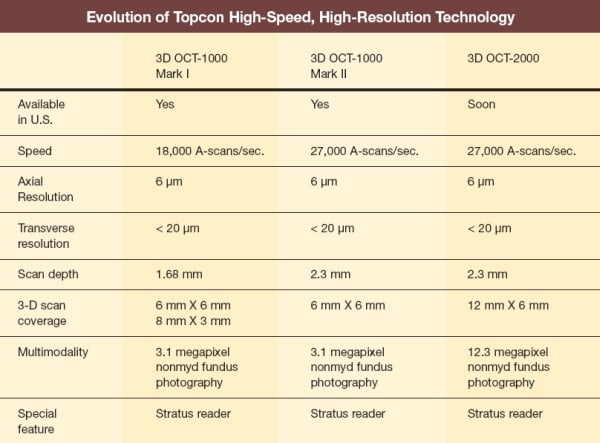
THE NEW STANDARD IN CLINICAL UTILITY
The 3D OCT-2000 from Topcon Medical Systems is an example of an SD-OCT instrument that fulfills these criteria but also provides the ophthalmologist with several additional practical benefits for a real-world efficient clinical practice.
• The 3D OCT-2000 is the only high-speed, high-resolution spectral domain option that is combined with a true color fundus camera (Figures 7 and 8). The 12.3 megapixel camera is external and upgradeable. Because the entire system is built on a nonmydriatic camera platform, it performs well in hard to image patients such as those with small pupils or media opacity. Pin-Point Registration software aligns the OCT data to the fundus image and helps register OCT scans between visits.

Figures 7 and 8. The Topcon 3D OCT-2000 combines the latest spectral domain OCT technology with a high-resolution color nonmydriatic fundus camera. The spectrometer is inside the body of the system, giving it a compact footprint.
• In addition to the 12.3 megapixel camera, the system includes a wide range of data capture protocols, including the most recently added 12 mm X 6 mm OCT scanning pattern. With this one large scan, a clear image of the optic nerve head, fovea and surrounding area is attainable (Figure 9).

Figure 9. With the Topcon 3D OCT-2000, a large 12 mm X 6 mm box scan can be used to image the optic nerve head, fovea and surrounding area.
• Retinal layer segmentation, including identification of the internal limiting membrane, nerve fiber layer, IS/OS junction, retinal pigment epithelium and Bruch's membrane, is among the many automated functions that are part of the system.
• A feature of the 3D OCT-2000 that we have found very useful is the ability to rapidly change the instrument's zone of maximum sensitivity from the vitreous to the choroid to enhance imaging of the choroid without needing to capture "inverted" images.
• Automated 3D OCT and fundus image capture technology and report functions make data acquisition fast and easy for all staff members, including those with limited prior training. From the system's large color touch screen, the user can move fixation, change scan patterns, autofocus and review images. It took less than 5 minutes to teach first-year residents and non-photography technicians to capture good quality images.
The instrument's ease of use can be especially appreciated in satellite clinics where staffing with professional ophthalmic photographers often is not feasible.
• For image viewing and evaluation, including comparing serial exams and applying analysis tools, the software is robust. The images load rapidly. It typically requires approximately 10 seconds per eye to scroll through the adjacent B-scans, and that approach makes interpretation of findings easier. With experience, the user's eye becomes trained to detect abnormalities while rapidly scrolling through images.
Also, 3-D renderings of the retina can be called up quickly. We have found those images, and the speed with which we can bring them to the screen, to be invaluable for patient education.
• The 3D OCT software includes a Stratus reader so that the practice can import all of its legacy Stratus scans into the new system. Maintaining a singe database obviously is preferred.
IMAGE AND DATA MANAGEMENT COMPLETE THE PICTURE
The networking solutions for 3D OCT-2000 are a major factor in a practice's ability to harness the power of the technology in a clinically practical fashion. With IMAGEnet software for image capture, processing and storage, up to 10 review stations can be linked without the need for an additional server.
The SD OCT-2000 also integrates with the EyeRoute ophthalmic image management system (Topcon Medical Systems Inc.). EyeRoute integrates images and reports from diagnostic instruments into a single, secure digital environment. Devices from most manufacturers, not just Topcon, can be included in the system. The system is also compatible with most electronic medical records packages.
EyeRoute is Internet browser-based, which means it eliminates the need for review station software from multiple manufacturers. Technicians export the data from each patient test into EyeRoute simply by pressing a button. From that point, everyone who needs to access the images and reports can do so from any computer — in the office or around the world. Even in low bandwidth environments, data can be displayed and analyzed and images from different modalities can be simultaneously viewed at a clinically relevant speed. Consulting and referring doctors can be given access to the system as well.
THE FUTURE OF OCT
SD-OCT instruments represent the future. They also represent a new standard of care for diagnosis and management of patients with retinal disorders. The Topcon 3D OCT-2000 is an excellent SD-OCT instrument that combines the exceptional image quality and performance expected from this technology with unique practical attributes that allow seamless integration into a busy, real-world clinical practice. RP
Dr. Sadda is an associate professor of ophthalmology at the Keck School of Medicine of the University of Southern California, Doheny Eye Institute. He is director of the Medical Retina Unit, Ophthalmic Imaging Unit, Retinal Cell Replacement Laboratory and Doheny Image Reading Center. Dr. Sadda is a consultant to Heidelberg Engineering, receives research support from Carl Zeiss Meditec and Optovue, and shares in royalties from intellectual property licensed to Topcon Corporation by the Doheny Eye Institute.
Dr. Walsh is an assistant professor of ophthalmology at the Keck School of Medicine of the University of Southern California and a member of the medical retina service at the Doheny Eye Institute. He also directs the Doheny Imaging Exploration and Software Engineering Laboratory (DIESEL). Dr. Walsh shares in royalties from software licensed to Topcon Corporation by the Doheny Eye Institute.
REFERENCE
1. Keane PA, Bhatti RA, Brubaker JW, Liakopoulos S, Sadda SR, Walsh AC. Comparison of clinically relevant findings from high-speed fourier-domain and conventional time-domain optical coherence tomography. Am J Ophthalmol. 2009;148:242-248.








