RPS: From the Podium to the Practice
Bringing Dry AMD Into Focus
A recap of dry AMD research and analysis presented at our 2009 annual meeting.
ANDREW E. MATHIS, PhD, MEDICAL EDITOR
In our last issue, we recapped presentations from the 5th Annual Retinal Physician Symposium, held earlier this year in the Bahamas, that dealt largely with the treatment of exudative AMD. In this issue, we turn our attention to dry AMD. Three experts in the field discussed the pathogenesis, progression, and treatment of varying cases of dry AMD. Here are the highlights.
ALLEN HO ON PATHOBIOLOGY, GENETICS, COMPLEMENT AND INFLAMMATION
Allen C. Ho, MD, of the Wills Eye Institute in Philadelphia, gave a lecture on the pathobiology, genetics, and role of the complement cascade, and inflammation in dry AMD. He began by talking about the AREDS trial. "If you think about it," he said, "this study gives us hope because AMD is a disease that is progressing over decades. We simply introduce some oral supplements for these patients well into their disease and actually have some kind of modest positive effects. It gives us hope that things that we try for patients with advanced dry AMD might be helpful."
Dr. Ho showed a fundus photograph (Figure 1) of a patient with multiple drusen to discuss pathobiology, followed by a cartoon depicting RPE cells in "intimate contact" with multiple photoreceptor cells, to emphasize the damage drusen may leave in terms of atrophy. He pointed out that RPE cells are charged with not only delivering vitamin A to the photoreceptors, but also taking and ingesting the receptor�s outer segments so that the photoreceptors can "remain new." Dr. Ho demonstrated this process with another animated slide and a detailed description of vitamin A metabolization in the eye.

Figure 1. The pathobiology of drusen.
Dr. Ho continued, "With time, what happens is that the RPE cells naturally begin to become overwhelmed with some of the cellular debris and vitamin A metabolites." The worst metabolite in this process, he said, is A2E, a metabolite that causes autofluorescence and that, when present in excessive levels, causes loss of photoreceptors and choroidal vessels, leading to geographic atrophy. Dr. Ho pointed out that, because patients with geographic atrophy may still have 20/20 vision, other methods of evaluation have to be considered.
To demonstrate how the changes over the natural history of geographic atrophy occur, Dr. Ho presented several images over time using fundus photography, fundus autofluorescence photography, and fundus microperimetry (Figure 2). "If you could stop that atrophy or stop the source of that atrophy," Dr. Ho said, "maybe you could have a treatment for dry atrophic AMD."
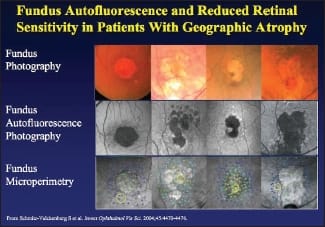
Figure 2. Comparative imaging of geographic atrophy.
Dr. Ho turned to discussing Sirion Therapeutics' investigational drug fenretinide, which had previously failed to prove efficacy in clinical trials for breast cancer, but which has a strong binding effect on excess vitamin A in the eye — thus perhaps offering the possibility of halting the progression of geographic atrophy. He gave details of a phase 2 trial of fenretinide that is under way.
Dr. Ho then returned to the subject of A2E, which he noted is not only toxic to RPE cells, but has also been shown to activate the complement cascade. Dr. Ho said, "If you increase exposure to A2E over time, you increase complement activity in systems." This is particularly relevant with regard to dry AMD in the inflammatory process and complement activity relevant to that process. A2E is one link to the role of inflammation and specifically the complement cascade in AMD.
To demonstrate, Dr. Ho presented the inflammatory process in dry AMD as described by Gregory S. Hageman, PhD, of the University of Iowa, in a paper published in 2001 in Progress in Retinal and Eye Research. Pointing to thickening of Bruch's membrane and basal lamina, Dr. Ho said, "This is the histopathologic hallmark of dry AMD. You get the inflammatory cells being attracted by the cytokines that are released. They extend processes through porous Bruch's membrane and create a drusen inflammatory core."
Mentioning that "a number of products are looking at this," Dr. Ho turned to Othera's investigational drug OT-551, which acts via multiple pathways and indirect activity against inflammation. However, Dr. Ho noted that the studies on OT-551 have, at this time, produced no evidence for efficacy.
Dr. Ho then turned to other complement systems, specifically complement factor H (CFH). "Complement factor H polymorphisms represent an unchecking of the activity of inflammation, and this is a way to conceptualize what a complement system and its proteins may do to a bacterium, causing inrushing of fluids and lysis of the bacterium," Dr. Ho said. When complement factor H mutates, it represents disregulation of inflammatory control which appears to play a role in the progression of AMD.
Dr. Ho turned to behavior controls next, pointing out that patients must modify those things they can control, such as smoking, diet, etc., rather than things they cannot modify, like genetics. "I've shown this slide for about five years but it still applies," he said, showing pictures of modifiable risk factors for AMD.
Among the modifiable factors are the dietary factors, and Dr. Ho next discussed lutein, zeaxanthin and omega 3 fatty acids. He talked about dietary sources of these compounds, as well as their inclusion in AREDS vitamins. Dr. Ho also looked at DHA and EPA before turning to questions from the audience, which focused on questions concerning AREDS vitamins' efficacy. Dr. Ho conceded that there are "non-AREDS believers," but he said, "I think because the cost is relatively low, although the benefit is modest, I am a believer."
DAVID BOYER ON TREATMENTS IN THE PIPELINE
David Boyer, MD, spoke next. Dr. Boyer, who is clinical professor of ophthalmology at the Keck School of Medicine at the University of Southern California and practices with Retina Vitreous Associates Medical Group in Beverly Hills, gave a presentation entitled "Dry AMD: Treatments in the Pipeline." Beginning with Dr. Carmen Puliafito's maxim that "dry AMD is the new wet AMD," Dr. Boyer explained that the attention being paid to the development of dry AMD treatments is comparable to the attention paid to wet AMD before anti-VEGF drugs came on the scene.
Dr. Boyer noted the much higher incidence of dry AMD (80% to 90% of patients in retinal practices seeking treatment for AMD) vs wet AMD (10% to 15%), also mentioning that about 3.5% of patients over the age of 75 have geographic atrophy that severely compromises their vision. Retinal physicians generally recommend AREDS vitamins, but, Dr. Boyer conceded, they do not really affect the progression of geographic atrophy. He maintained that it is primarily the zinc in the AREDS formulation that was of the greatest importance and relevance. However, Dr. Boyer continued, nutrition and lifestyle modifications are important.
Then Dr. Boyer turned to investigational drug treatments. "The biggest problem is looking at endpoints," he said, turning to the different studies he covered in his presentation. "When you look at endpoints to see whether a drug is successful, what are we really looking at? Are we going to look at vision? Are we going to look at reading speed, or scotoma size?"
He demonstrated the difficulty in rating efficacy by showing a slide from Janet Sunness, MD, demonstrating how little change occurs in cases of geographic atrophy over a two-year period (Figure 3). Another slide adapted from work by Frank Holz, MD, demonstrated that fundus autofluorescence can be a bit more effective in tracking progression. It is clear, however, that using vision as an endpoint is not practical.
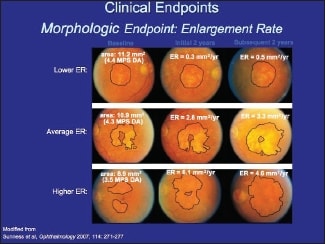
Figure 3. Changes in geographic atrophy over a two-year period.
Dr. Boyer then discussed the first drug on his list, OT-551, which Dr. Ho had covered in his own presentation. Dr. Boyer dealt first with the administration method; because it is an eyedrop, many people are skeptical of OT-551's ability to effect change on the retina, but he pointed out that, at least in rabbit eyes, OT-551 was effective in getting to the back of the eye. Turning to OT-551's mechanism of action, Dr. Boyer conceded that it is not fully understood, but he suggested that, by reducing oxidative stress, OT-551 may reduce inflammation. One possible use of OT-551, he noted, might be as an adjunct to ranibizumab.
Deferring to Dr. Ho on fenretinide, except to point out some adverse effects due to vitamin A blocking, Dr. Boyer turned to Copaxone (Teva Pharmaceuticals's brand of glatiramer acetate), a drug that is already approved by the FDA for use in multiple sclerosis and investigationally in Alzheimer's disease. Scientists in Israel found that it could affect drusen.
According to Dr. Boyer, Copaxone works by increasing the proportion of T-helper-2 lymphocytes, which modulate inflammation. He turned to several slides of patients with dry AMD treated with Copaxone, making reference to the children's book Where's Waldo? by asking the audience to identify the areas of reduced drusen after 12 weeks. In several patients, the amount of drusen was reduced in the 12-week period since baseline, indicating possible efficacy for Copaxone in treating dry AMD (Figure 4).
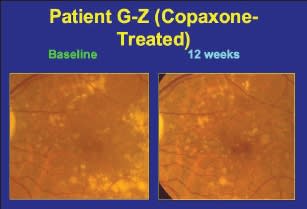
Figure 4. Baseline and 12-week images of an eye treated with Copaxone.
The next drug discussed was an intravitreal implant that dispenses the antiglaucoma drug brimonidine (Allergan, Irvine, CA) in a sustained release. Dr. Boyer compared it to Allergan's Ozurdex dexamethasone implant in terms of how it works, and discussed a trial of 116 patients that had just completed enrollment, noting that brimonidine seems to work as a neuroprotectant, activating a number of pathways, both inside and outside cells. He noted that it is also under investigation for treatment of glaucoma, albeit at a much higher dose and for a longer period of time.
Dr. Boyer then turned to the GA Trial, an extension of the GAP trial that Alcon had sponsored. The drug being investigated, he said, is a selective serotonin agonist that seems to induce resistance to oxidative stress. "It seems to have a very good track record in animals to be able to do this," Dr. Boyer said, "and it does possibly have the potential to reduce apoptosis."
Dr. Boyer closed his presentation with a short overview of proposed treatments for dry AMD that have failed. "We've had laser to drusen, which did show drusen will decrease," he said. "They will go away, but over the long term, in two separate trials, there was really no improvement in vision." He also noted the failure of rheopheresis as a treatment for dry AMD, but he expressed hope that interim results from fenretinide and OT-551 would furnish more positive results.
JASON SLAKTER ON COMPLEMENT INHIBITION
The last presentation on dry AMD was by Jason S. Slakter, MD, of Vitreous-Retina-Macula Consul tants of New York. Dr. Slakter presented information on complement inhibition and whether this approach could offer new opportunities in treating dry AMD. The objectives he laid out for his talk included explaining the rationale for complement inhibition, recalling the questions that still remain regarding delivery, timing, and risks, and then naming the first complement inhibitor undergoing evaluation in clinical trials for the treatment of dry AMD.
Dr. Slakter began by saying, "Retina people have a habit of taking things and doing it to the eye to see whether it works, and then going back to the laboratory and trying to explain 'How did that work?'" He gave macular hole surgery as an example of this trend. However, he noted, complement inhibition is one of the few things where, he said, "we did things the right way."
Dr. Slakter noted that, before April 2005, there was little or nothing in the medical literature about complement inhibition and the eye, and then three publications appeared in Science and one in the Proceedings of the National Academy of Sciences in a single month, detailing genetic studies that link abnormalities in complement factor H and AMD. Since then, he said, publications on complement dysfunction in ophthalmic journals have been regular. "Certainly," Dr. Slakter said, "complement has been the strongest genetic factor associated with AMD and it led us to take a look at it as a potential therapeutic target."
This led to the question: What is complement? Dr. Slakter explained that it is part of the immune system, saying, "Complement plays a protective role; the complement pathway generates multiple factors that attack and kill foreign organisms and abnormal cells." He pointed out, however, that there must be a way to regulate the process to prevent uncontrolled inflammation.
Dr. Slakter then presented a slide showing the three different pathways that converge and result in complement activation, indicating where in the complement cascade intrinsic controls are in place — specifically where complement factor H plays a key role. He noted that factor C3 is particularly problematic, as it plays a role in an amplification loop that can get out of control. CFH, according to Dr. Slakter, "tamps down the system and prevents it from getting out of control." With abnormal CFH, such controls are diminished and excess inflammation may result.
"What we have is a pretty compelling story," Dr. Slakter said. "In addition to the genetic findings, research has shown that drusen have complement and CNV models have shown complement to be present as well. So we have a mission — Let's go out and block complement." The problem, he noted, is that there are still many things we don't know about complement, ie, the source of complement activation, pathway involvement, lifelong vs acute over-reactivity, therapeutic benefits, and risks of complement inhibition.
Nevertheless, Dr. Slakter noted, the discovery of the relationship between AMD and the complement system is exciting because, if complement is involved in drusen formation and CNV, then there may be a way to control neovascularization as well as to prevent the progression of geographic atrophy. It may be the "silver bullet" for treating all forms of AMD.
Dr. Slakter then showed where four therapeutic agents under investigation enter the complement cascade. He spoke briefly about two of the agents, Jerini's JPE1375 and Oph thotech's ARC-1905 before speaking at length about POT-4, which Dr. Slakter described as "a compstatin derivative with a much higher affinity to complement." He noted POT-4's stability and remarked further that what distinguishes this drug from others in its impact on the complement system is that it binds to C3 but does not kill it (Figure 5). This stops the amplification loop, but the body still keeps C3 in circulation.
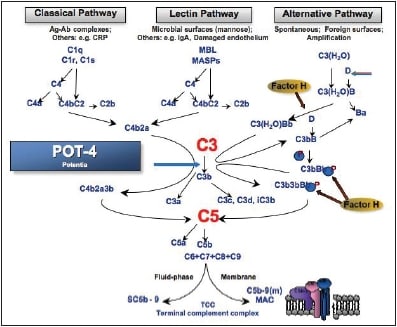
Figure 5. The complement pathway and the location where POT-4 intervenes.
To demonstrate the efficacy of POT-4, Dr. Slakter presented in vitro and in vivo models, including an E. coli model in baboons, where there was complete complement inhibition. However, he noted, complement is the molecule is species-specific, so he turned to human studies. He detailed the results of the ASaP phase 1 trial of POT-4, where the drug was tested in patients with late-stage wet AMD by administering a single injection. In presenting the phase 1 data, he was careful to warn the audience that there was still no evidence that POT-4 works, even though the safety profile was very positive.
He then discussed how it was discovered that POT-4 has a unique property: It forms a large blob in the vitreous after injection that slowly dissolves over time and slowly releases the drug over time (Figure 6). In humans, the dose at which this first occurs is 450 mg. Dr. Slakter showed graphs of serum levels of POT-4 in patients and halflives of the drug, suggesting the possibility that at higher doses the drug may remain at therapeutic levels for up to six months with a single injection.
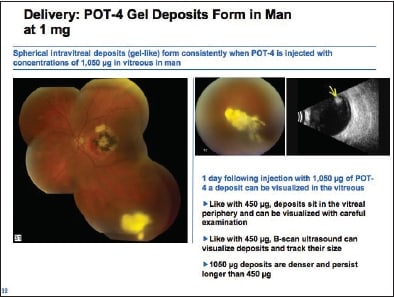
Figure 6. POT-4 gel deposit in the human eye.
Dr. Slakter closed his presentation with an anecdote. Philip Rosenfeld, MD, of the Bascom Palmer Eye Institute in Miami, had sent to Dr. Slakter the previous day images from a patient who had 20/800 vision and had an OCT image that showed almost total resolution of the retinal edema and fluid after one month. "Does that prove anything? A lucky patient, maybe," Dr. Slakter said, underscoring the importance of proceeding with caution in drawing conclusions without phase 2 and phase 3 data.
WHAT'S NEXT
In the next issue of Retinal Physician, our first for 2010, we'll conclude our coverage of the 2009 Retinal Physician Symposium, with an eye toward looking forward to 2010's symposium. RP








