Vitreous Floaters: When a Minor Nuisance Becomes a Serious Issue
Floaters can be caused by a variety of conditions, from benign to vision threatening.
PHILIP J. POLKINGHORNE, MD, BSc, FRCOphth, FRANZCO
A patient presenting with floaters is, for most retinal surgeons, an opportunity to exclude the existence of a retinal break and or retinal detachment. Less frequently, floaters coexist with other posterior-segment pathologies such as vitreous hemorrhage, inflammatory eye syndromes, and optic nerve disease.1 In most cases, the etiology of the vitreous opacities can be diagnosed on clinical grounds, although on occasion, further investigations such as fluorescein angiography, ultrasonography and rarely, vitreous biopsy are indicated. The essential feature for investigating patients with floaters is the atypical history and or examination.
Patients presenting with floaters in the absence of retinal pathology are usually reassured with advice, but an unknown minority are intolerant of their symptoms and request intervention. Anecdotally, patients who appear most troubled by floaters are those with evidence of a posterior vitreous detachment (PVD). Not all the patients in this group have a Weiss ring, suggesting that combinations of otherwise simple separation of the posterior hyaloid and patterns of vitreous collapse may, in certain individuals, be sufficient to cause significant visual symptoms. Patients who are highly myopic and under 60 also appear to be over-represented in the intolerant group, as are young pseudophakes.
The enigma for the retinal surgeon evaluating such patients is the inability to quantify or measure the degree of visual impairment. Indeed, it is likely the patient will have a normal acuity for both distance and near. The relationship between what is apparent in the vitreous cavity on slit-lamp biomicroscopy and the patient's symptoms is at best poor. Furthermore, the utility of ultrasound and OCT is also restricted. The limitation of ultrasound in detecting small vitreous opacities relates to the inability to separate background noise from the reflected echo. In short, the acoustic interface between the vitreous gel and vitreous opacity is simply too small. This differential, that is the acoustic impedance, may reach threshold if the density of the individual vitreous particles is increased or there is sufficient number of particles or interfaces. This explains why it is possible to visualize some vitreous opacities such as asteroid hyalosis and blood in the vitreous cavity (Figure 1).

Figure 1. Ultrasound image of an eye with asteroid hyalosis.
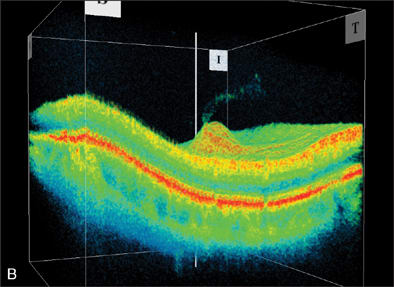
The spatial resolution of ultrasound is dwarfed in comparison with the OCT. However, OCT also depends on impedance, albeit using a coherent light source, so the limitations described for ultrasound also apply for OCT. Modern spectral-domain OCTs have the ability to acquire images within a relatively short timeframe and can image certain vitreal structures but usually not discrete opacities (Figures 2 and 3). There are other laboratory-based modalities available to measure the density and distribution of small particles or suspended matter in a liquid environment, but to date these approaches have not reached clinical practice.2

|
|
Figure 2. Optical coherence tomography image detailing vitreous separation from the optic nerve head.

Figure 3. OCT image detailing vitreous separation from the optic nerve head.
The inability to correlate symptoms with vitreal opacities has not prevented investigators from devising procedures to lessen or remove the obscurations. Unfortunately, there are no natural history studies detailing outcomes without intervention, so the literature bias is weighted toward treatment options. For most retinal surgeons the procedure of choice is a pars plana vitrectomy. This approach is at least in part supported with well-documented improvements in quality of life indices, which include both vision-specific and general well-being indicators.3
Vitreolysis with the Nd-YAG laser has also been advanced as a useful tool for the treatment of troublesome floaters. Tsai et al. reported on 15 eyes treated with the Nd-YAG laser and documented an immediate resolution of symptoms with a single treatment session.4 Other authors have also reported on the efficacy of Nd-YAG vitreolysis although it is recognized multiple treatment sessions are sometimes required.5 The treatment strategy for the large solitary floaters is to target the obscurations directly with the aim of disrupting the floater into small fragments. An indirect approach is usually advocated for the smaller, multiple floaters with high-energy bursts titrated to promote further collapse of the vitreous matrix.5
Delaney et al. compared the outcomes of Nd-YAG vitreolysis and vitrectomy in a series of 31 patients presenting with symptomatic floaters.5 The former technique ameliorated symptoms in one-third of patients, although 3 patients reported a worsening of their symptoms following treatment. The results with pars plana vitrectomy were better and, of the 16 eyes that underwent surgery, there was complete resolution of symptoms in all but 1 eye. This arm of the study also included 11 eyes that had unsuccessful Nd-YAG vitreolysis. Response to treatment, however, is not the only outcome measure, and factoring risk as well as the cost of intervention is required in today's environment. The short- and long-term risks of both Nd-YAG vitreolysis and vitrectomy include injury to adjacent structures resulting in cataract, retinal detachment, and cystoid macular edema.6 Additionally, there is always the risk of endophthalmitis with vitrectomy surgery and the recent reports of adverse biochemical changes in the vitrectomized eye provide impetus for exploring alternative treatment strategies.7
One such approach might involve pharmacologic vitreous disruption. Such a drug would be delivered via intravireal injection, and there is a body of evidence governing this technique with established guidelines detailing both volumetric dose limitations and infection control.8-10
For pharmacologic vitreous disruption to be successful, 2 events would need to occur in the eye. The first would be adequate liquefaction of the vitreous gel (synersis). The second would be separation of the posterior hyaloid from the retina (synchisis) in a controlled fashion. As yet, it is not possible to either predict or discern what alteration in the vitreous gel causes an individual patient to become intolerant of his or her vitreous opacities. Some patients are intolerant of floaters with an attached vitreous, whereas others are only significantly troubled after the vitreous has separated. Conceivably, different treatment strategies might be dictated by the status of the posterior hyaloid.
Currently, most therapeutic endeavors to alter the vitreous gel are based on an enzymatic approach.11 Gandorfer has recently reviewed this subject and evaluated the potential application for a variety of sight-threatening vitreoretinal pathologies.12 The candidate enzymes for both syneresis and synchisis include chondroitinase, dispase, plasmin, and hyaluronidase. These enzymes have varying degrees of activity against component macromolecules within the vitreous gel and at the vitreoretinal interface. The substrate for chondroitinase is chondroitin sulphate, which is integral in the maintenance of the vitreoretinal interface. Dispase is a protease that disrupts collagen and in the vitreous cavity has a propensity for type 4 collagen, which is also found at the vitreoretinal interface. Plasmin, a nonspecific protease, is also active at the vitreoretinal interface hydrolyzing the glycoproteins laminin and fibronectin.11 The transparency of the vitreous depends on the molecular structure of the vitreous. To ensure there is only minimal scattering of light, the collagen fibrils in the vitreous gel need to be spaced by at least 1 wavelength of visible light. Hyaluronin is an important macromolecule in maintaining the collagen spacing, so hydrolyzing this molecule with hyaluronidase has the potential to induce syneresis, which could relocate any vitreous opacities away from the visual axis.
The translation from the in vitro laboratory studies to the clinical arena for these candidate enzymes thus far has proved disappointing. Only chondroitinase and hyaluronidase have been tested in formal clinical trials and neither achieved approval for the clinical applications under review. Plasmin has been used in uncontrolled studies as an adjunct to vitrectomy surgeries13,14 and, more recently, as a treatment modality for the treatment of diffuse DME.10 In these situations, plasmin was thought to offer a therapeutic advantage in separating the posterior hyaloid facilitating the surgery, and in the eyes with diffuse diabetic macular edema, an improvement in visual acuity and a reduction in macular thickness was noted.10
A significant drawback to the use of plasmin relates to its biological instability: There are difficulties in preparation from the donor and in determining the biological activity of the aliquot. Recombinant technology may overcome this problem and the development of microplasmin, a biologically active derivative of plasmin, might prove a way forward.15 In vitro studies with microplasmin in porcine vitreous specimens induced a dose-dependent alteration in the size macromolecules in the vitreous, as well as a dose-dependent separation of the posterior hyaloid.11,16 Furthermore, the safety profile of recombinant microplasmin at therapeutic doses in rabbit eyes do not induce any retinal ultrastructural changes and no long-term ERG abnormalities were observed.16 There were, however, some transient ERG changes noted during the first week following treatment.
Further studies are obviously needed to evaluate the safety and efficacy of microplasmin but the early indication is that this drug is emerging from the realm of possibility to probability. How-ever, it is salient to heed Sebag's warning that advances in pharmacologic vitreous disruption need to be matched with progress in understanding the physiology and pathophysiology of the vitreous.11 Unless the molecular basis of interaction is known, outcomes may precipitate adverse events like anomalous vitreous separation, macular pathologies, and retinal breaks. The hope is that manipulating the structure of the vitreous may provide an alternative treatment options for a wide range of vitreoretinopathies, including vitreous opacities. RP
| Philip J. Polkinghorne, MD, BSc, FRCOphth, FRANZCO, is associate professor in clinical ophthalmology at the University of Auckland, New Zealand. He reports no financial interests in any products mentioned here. He can reached via e-mail at pjpolk@pjpolk.co.nz. |
REFERENCES
- Roufail ED, Polkinghorne P. Vitreous floaters. Compr Ophthalmol Update. 2006;7:171-177.
- Ansari RR. Ocular static and dynamic light scattering: a noninvasive diagnostic tool for eye research and clinical practice. J Biomed Opt. 2004;9:22-37.
- Schiff WM, Chang S, Mandava N, Barile GR. Pars plana vitrectomy for persistent, visually significant vitreous opacities. Retina. 2000;20:591-596.
- Tsai WF, Chen FC, Su CY. Treatment of vitreous floaters with neodymium YAG laser. Br J Ophthalmol. 1993;77:485-488.
- Delaney YM, Oyinloye A, Benjamin L. Nd:YAG vitreolysis and pars plana vitrectomy: surgical treatment for vitreous floaters. Eye. 2002;16:21-26.
- Little HL, Jack RL. Q-switched neodymium: YAG laser surgery of the vitreous. Graefes Arch Clin Exp Ophthalmol. 1986;224:240-246.
- Stefansson E. Physiology of vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2009;247:147-163.
- Ladas ID, Karagiannis DA, Rouvas AA, et al. Safety of repeat intravitreal injections of bevacizumab versus ranibizumab: our experience after 2,000 injections. Retina. 2009;29:313-318.
- Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90:1344-1349.
- Díaz-Llopis M, Udaondo P, García-Delpech S, et al. [Enzymatic vitrectomy by intravitreal autologous plasmin injection, as initial treatment for diffuse diabetic macular edema.] Arch Soc Esp Oftalmol. 2008;83:77-84.
- Sebag J. Molecular biology of pharmacologic vitreolysis. Trans Am Ophthalmol Soc. 2005;103:473-494.
- Gandorfer A. Enzymatic vitreous disruption. Eye. 2008;22:1273-1277.
- Williams JG, Trese MT, Williams GA, Hartzer MK. Autologous plasmin enzyme in the surgical management of diabetic retinopathy. Ophthalmology. 2001;108:1902-1905; discussion 1905-1906.
- Margherio AR, Margherio RR, Hartzer M, et al. Plasmin enzyme-assisted vitrectomy in traumatic pediatric macular holes. Ophthalmology. 1998;105:1617-20.
- Nagai N, Demarsin E, Van Hoef B, et al. Recombinant human microplasmin: production and potential therapeutic properties. J Thromb Haemost. 2003;1:307-313.
- Sakuma T, Tanaka M, Mizota A, et al. Safety of in vivo pharmacologic vitreolysis with recombinant microplasmin in rabbit eyes. Invest Ophthalmol Vis Sci. 2005;46:3295-3299.








