Central Serous Retinopathy
MICHAEL COLUCCIELLO, MD
Central serous retinopathy (CSR; ICD-9: 362.41) is an exudative chorioretinopathy characterized by an exudative neurosensory retinal detachment with or without an associated detachment of the retinal pigment epithelium (RPE). This disorder results in metamorphopsia and micropsia. The disease was first recognized by Albrecht von Graefe in 1866, who termed the condition "idiopathic detachment of the macula" and "central recurrent retinitis."1 Perhaps most appropriately designated a choroidopathy, it is currently most commonly termed a retinopathy.
EPIDEMIOLOGY
Most cases of the CSR occur in individuals between 20 and 50 years of age. One study found the age range at first diagnosis to be between 22 and 83 years old. In that study, patients older than 50 tended to have bilateral disease, systemic hypertension, and a history of corticosteroid use.2 The overall incidence in men vs women is approximately 6 to 1. In 1 study, mean annual age-adjusted incidences per 100000 were 9.9 for men and 1.7 for women.3 In women, pregnancy is a known risk factor.4
CSR has been associated with stress and stress hormones (ie, corticosteroids and epinephrine); individuals with a "type A personality" who are under stress have been shown to be predisposed to developing the condition.5 There is extensive evidence that those exposed to exogenous (even topical) corticosteroids (eg, those with asthma, autoimmune disorders, dermatologic conditions, allergic rhinitis, degenerative disc disease requiring epidural steroids, and organ transplant patients) or higher levels of endogenous corticosteroids (Cushing syndrome) or epinephrine (obstructive sleep apnea,6 systemic hypertension) show a higher frequency of CSR.6 In 1 series, 52% of patients with CSR had used exogenous steroids within 1 month of presentation as compared to 18% of control subjects.7 The incidence of CSR in persons with Cushing syndrome was 5% — far higher than the general population — in 1 small study.8 Sildenafil (Viagra, Pfizer) usage has also been linked to the development of CSR.9,10
The incidence of recurrence in the ipsilateral eye is approximately 30%; in 1 study the incidence of signs of CSR in the fellow eye was 32% (symptomatic in 13%).11 One study associated Helicobacter pylori infection with CSR;12 no further studies have corroborated this finding.
| Michael Colucciello, MD, is a clinical associate in the Department of Ophthalmology at the University of Pennsylvania School of Medicine in Philadelphia and a retina specialist practicing with South Jersey Eye Physicians in Moorestown, NJ. He reports no financial interests in products mentioned in this article. Dr. Colucciello can be reached via e-mail at michael@macula.us. |
PATHOPHYSIOLOGY
Several hypotheses have been offered regarding the pathogenesis of CSR, but none have been definitively proved. Hypotheses have been offered based on epidemiologic and angiographic observations.
Patients with CSR may have an increased susceptibility to choroidal and RPE hyperpermeability with steroids or epinephrine. Impairment in autonomic function may lead to a focal disturbance/spasm in the choroidal circulation.13
In these patients, choroidal vasoconstriction mediated by epinephrine and potentiated by corticosteroids may lead to choroidal ischemia and hypermeability.14,15 The exudation of plasma proteins results in increased oncotic pressure in the extravascular space, inducing a malfunction of the RPE pump mechanism and disruption of the zonula adherens between RPE cells, leading to an RPE leak.16 This disruption of the outer blood-retinal barrier then allows for transudate between the RPE and retina resulting in a neurosensory retinal detachment.17-19 Damage to active fluid transport mechanisms in the RPE (normally dehydrating to the subretinal space) may also play a role in the process.20
CLINICAL PRESENTATION
Patients present with an acute onset of central scotoma, metamorphopsia, and micropsia (detected by Amsler grid). Visual acuity (VA) is often improved with a small hyperopic correction. There is an decrease in contrast sensitivity and color saturation and an increase in macular photostresss recovery time.
Contact lens biomicroscopy demonstrates a serous macular neurosensory retinal detachment without blood. Often, subretinal deposits are noted as "dots" in the area of the serous detachment (Figure 1). RPE detachments, retinal pigment epithelial mottling and atrophy (sometimes in a "gutter" configuration), and subretinal fibrin (especially when the episode occurs during pregnancy) may also be seen. Uncommonly, subretinal lipid is seen in CSR.
EVALUATION
Studies complementary to clinical examination offer information leading to the diagnosis of CSR, allow for discrimination in the differential diagnosis (Table 1), and give data helpful in deciding possible therapeutic options.
| Table 1. Central Serous Retinopathy Differential Diagnosis |
|---|
Choroidal Neovascularization due to:
Idiopathic Serous RPE Detachment Vogt- Koyanagi- Harada Disease Macular Hole Optic Nerve Pit RPE Dystrophy (eg, Best) Malignant Hypertension Choroidal Tumors
Acute Lymphocytic Leukemia |
Optical coherence tomography (OCT) (Figure 1 inset) demonstrates many of the signs listed above, most notably the presence of a neurosensory macular detachment, which may be subtle. Intravenous fluorescein angiography (IVFA) in CSR demonstrates focal leakage at the level of the RPE. Pinpoint leakage sites are usually seen; a "classic smokestack" configuration of fluorescein leakage (Figure 2) is seen in only 10% of cases. In chronic CSR, a focal granular hyperfluorescence at the level of the RPE can be seen, associated with areas of atrophy of the RPE.
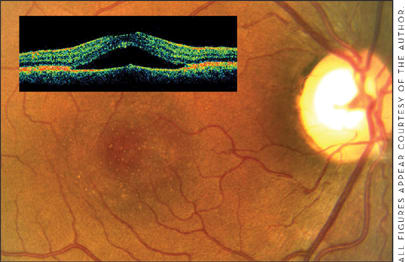
Figure 1. Clinical biomicroscopic presentation of CSR: serous macular detachment, with punctuate subretinal deposits in the region of the neurosensory detachment. Inset: OCT shows macular detachment in the same patient.

Figure 2. Sequential IVFA images (to 6 minutes, right) demonstrate classic "smokestack" leakage at the level of the RPE with pooling of dye in the sub-neurosensory retinal space in a patient with an acute episode of CSR.
Indocyanine green angiography (ICG) shows early hypofluorescence and late hyperfluorescence in areas of involvement. In very late phases, silhouetting of larger choroidal vessels is seen. ICG may also show subtle (often clinically inapparent) RPE detachments and areas of choroidal vascular hyperpermeability not detected with IVFA.
Multifocal electroretinography has been used to correlate the signs of CSR with regions of decreased retinal function and to monitor response to therapy. Microperimetry can measure retinal-sensitivity changes in CSR. Blood studies are generally not helpful, although elevated endogenous cortisol levels have been noted with CSR.
NATURAL HISTORY
CSR tends to resolve spontaneously within 8 weeks, with recovery of vision in approximately 90% of patients. Snellen VA may return to 20/20, but subtle metamorphopsia, nyctalopia, and dyschromatopsia may persist.
Approximately 30% of patients have recurrences;3 10% have 3 or more. Nearly half of those patients who experience a recurrence have that recurrence within 1 year of the initial presentation of CSR. Visual function may progressively decline with each recurrence. Retinal pigment epitheliopathy (decompensation of the RPE or "sick RPE syndrome"), cystoid macular degeneration (cystoid foveal changes on OCT without associated intraretinal leakage of fluorescein dye on IVFA), and CNV can complicate the process,15 especially with multiple recurrences.
DIFFERENTIAL DIAGNOSIS
Considerations for differential diagnosis in CSR include disorders that involve central vision loss associated with exudative neurosensory retinal detachment.
Neovascular exudative ("wet") age-related macular degeneration (AMD) presents with a neurosensory macular detachment. Although wet AMD presents in patients over the age of 50, CSR may also initially present in the same group. In wet AMD, in contrast to CSR, hemorrhagic neurosensory retinal detachments and hemorrhagic RPE detachments may be noted. Also, subretinal hard exudation is much more common in wet AMD than in CSR. Soft drusen and a familial history are characteristic of AMD. IVFA in neovascular AMD demonstrates CNV membranes — areas of leakage rather a focal point, as seen in CSR. The areas of early leakage on IVFA in wet AMD are typically lacy annular subretinal hyperfluoresence, seen in "classic" CNV, shallow fibrovascular RPE detachments with stippled hyperfluoresence, or a combination of the 2 conditions. ICG angiography in exudative AMD often shows a plaque of hyperfluoresence, rather than silhouetting of the larger choroidal vessels.
A variant of wet AMD, retinal angiomatous proliferation (RAP), often presents with intraretinal hemorrhage, which is not seen in CSR. IVFA in RAP demonstrates dilated capillaries near an area of intraretinal neovascularization that extends to the subretinal space and leaks. ICG in RAP shows retinal-retinal anastomosis and retinal leakage with a focal "hot spot" of leakage. Isolated serous or drusenoid RPE detachments in AMD present without contiguous exudation clinically or on OCT and do not demonstrate leakage on IVFA.
A macular neurosensory retinal detachment may be associated with other disorders that can lead to CNV. A multifocal choroiditis, such as presumed ocular histoplasmosis syndrome, may be associated with an exudative macular detachment in association with CNV. Fundus lesions typified by multiple choroidal lesions, with or without peripapillary atrophy, are associated with clinical and angiographic evidence of CNV. Degenerative myopia, in a highly nearsighted eye, may be associated with exudative CNV, especially when lacquer cracks are present. Angioid streaks may also be associated with exudative CNV. This condition may be discerned by the characteristic radial peripapillary linear dark red subretinal lines that represent regions of Bruch's membrane dehiscence.
Idiopathic serous RPE detachments, which typically occur in younger individuals and may be associated with metamorphopsia, are not associated clinically with serous retinal detachment upon examination with ophthalmoscopy or OCT and do not exhibit leakage on IVFA.
Vogt-Koyanagi-Harada (VKH) disease is a multisystem disease characterized by granulomatous uveitis with exudative retinal detachment associated with cutaneous, auditory, and neurologic manifestations. Distinguishing features include the presence of uveitis and disc edema associated with bilateral exudative retinal detachments, often involving the macula and inferior retina. Vitiligo, poliosis, meningeal signs with cerebrospinal fluid pleocystosis, focal neurologic signs, dysacusis, tinnitus, and vertigo are common systemic manifestations. There is a human leukocyte antigen DR4 genetic predisposition to the disease, encompassing Hispanic, Asian, Native American, and Middle Eastern populations. OCT examinations in VKH disease show an exudative retinal detachment as in CSR, but IVFA shows multiple pinpoint areas of RPE leakage, optic disc hyperfluoresence and retinal vascular leakage (Figure 3).

Figure 3. IVFA of right and left eyes in a patient with acute VKH syndrome, showing multiple pinpoint bilateral, symmetric RPE leaks associated with bilateral serous macular detachments. This patient, who exhibited HLA-DR4, also had vertigo, dysacusis, vitiligo, headache and a rash.
A macular hole may also be accompanied by a surrounding retinal detachment. A full-thickness foveal defect characterizes the condition. Approximately 50% of patients with optic nerve pits develop macular detachments. Congenital optic disc pits are typically hypopigmented oval or round defects averaging 500 μm (one-third of a disc diameter) in size, usually in the inferior temporal portion of the optic cup. RPE dystrophies, such as Best disease, typically appear with bilateral symmetrical foveal or parafoveal RPE pigmentary changes. Serous macular detachments can be associated with these conditions due to choroidal neovascularization. Electrooculography is diminished in Best's disease.
Vascular disorders, such as hypertension, may have an associated serous macular detachment. Choroidal tumors (hemangioma, metastasis, and melanoma) may also have an associated macular serous retinal detachment. Acute lymphocytic leukemia has been associated with serous macular detachments.16
TREATMENT OPTIONS
Since the prognosis for CSR is good; 80% to 90% of cases resolve spontaneously within 3 months. Observation is the first option in management. Certainly, if the patient is taking exogenous corticosteroids, these should be tapered and discontinued if possible.
If the patient is not taking exogenous steroids and the episode does not appear to be improving after 2 months, if previous episodes have left the patient with residual deficit or the visual symptoms are affecting the patient's activities of daily living to a significant extent, or if the patient needs to continue exogenous steroids for treatment of a medical condition, active treatment should be considered.
Photodynamic therapy (PDT) directed at RPE leaks may hasten resolution of exudation in CSR by reducing choroidal blood flow in those areas, thus favoring cessation of leakage. Since there is upregulation of vascular endothelial growth factor (VEGF) locally in areas of PDT,17 the development of CNV after PDT treatment of CSR is not surprising.18 "Safety-enhanced" PDT using half-dose verteporfin (Visudyne, QLT/Novartis), compared to the AMD treatment protocol, has shown promise.19
Laser photocoagulation of RPE leakage sites in CSR can also hasten resolution of leakage, resulting in resolution of associated serous macular detachment. Laser is only an option for extrafoveal leakage sites because collateral retinal damage follows laser treatment in all cases. Laser treatment was shown to be associated with a decreased CSR recurrence rate in 1 study.20 Potential complications include thermal rupture of Bruch's membrane with hemorrhage, secondary CNV,21 and misplaced laser spots.
Medical treatments have also been investigated: Acetazolamide given in a tapering dose for 6 weeks has been shown to reduce the time for subjective and objective CSR resolution, but it had no effect on final VA or recurrence rate.22 Most patients in the experimental group in that study had side effects from the acetazolamide, including paresthesias, nervousness, and gastric upset. Patients with sulfa allergies should avoid acetazolamide.
The beta adrenergic receptor blocker propranolol and the mixed alpha and beta adrenergic receptor blocker labetalol have plausible mechanisms of action in CSR if epinephrine is indeed involved in the pathogenesis of the disorder. Small studies have demonstrated evidence of efficacy.23,24
Mifepristone (Mifeprex, Danco Laboratories) is an antagonist of progesterone and glucocorticoid receptors; in a case study, 1 patient with CSR responded well to treatment with mifepristone.25 The antifungal ketoconazole is an adrenocorticoid antagonist that has been shown to lower endogenous cortisol; administration was associated with a decreased height of serous detachment after 8 weeks of treatment in 1 study.26
Intravitreal bevacizumab (Avastin, Genentech) has been investigated for treatment of CSR; theoretically, the antipermeability characteristics of this antibody to VEGF may allow for reduced leakage, favoring resorption of the exudative retinal detachment in CSR.27
A summary of treatment options appears in Table 2.
| Table 2. Treatments for Central Serous Retinopathy |
|---|
| Observation Surgical
Medical (Investigational)
|
CONCLUSION
CSR is characterized by an exudative macular detachment associated with focal points of RPE hyper-permeability, resulting in vision changes that include metamorphopsia and micropsia. Accurate diagnosis of CSR via clinical examination and ancillary studies to rule out differential diagnosis allows for appropriate counselling of the patient: With observation (and discontinuation of exogenous steroids, if applicable), the natural history is good. Treatment to hasten resolution of the serous macular detachment should be considered in patients with chronic cases, in individuals who need to be on a regimen of exogenous steroids, or in those persons with active CSR who have experienced some irreversible loss in vision with prior episodes. Treatment options include laser photocoagulation, "safety-enhanced" PDT, and a (growing) variety of medical treatments as indicated above. With hope, in the future, a more definitive picture of the pathogenesis will lead to a more specific treatment regimen and prophylaxis strategy for those predisposed to this condition. RP
REFERENCES
- von Graefe, A. Ueber central recidivierende retinitis. Graefes Arch Clin Exp Ophthalmol. 1866;12:211-215.
- Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103:2070-2079; discussion 2079-2080.
- Kitzmann AS, Pulido JS, Diehl NN et al. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology. 2008;115:169-173.
- Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;1111:244-249.
- Yannuzzi LA. Type A behavior and central serous chorioretinopathy. Trans Am Ophthalmol Soc. 1986;84:799-845.
- Carvalho-Recchia CA, Yannuzzi LA, Negrao S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109:1834-1837.
- Leveque TK, Yu L, Musch DC, et al. Central serous chorioretinopathy and risk for obstructive sleep apnea. Sleep Breath. 2007;11:253-257.
- Bouzas EA, Scott MH, Mastorakos G, et al. Central serous chorioretinopathy in endogenous hypercortisolism. Arch Opthalmol. 1993;111:1229-1233.
- Allibhai ZA, Gale JS, Sheidow TS. Central serous chorioretinopathy in a patient taking sildenafil citrate. Ophthalmic Surg Lasers Imaging. 2004;35:165-167.
- Fraunfelder FW, Fraunfelder FT. Central serous chorioretinopathy associated with sildenafil. Retina. 2008;28:606-609.
- Bujarborua, D, Chatterjee, S, Choudhury, A, et al. Fluorescein angiographic features of asymptomatic eyes in central serous chorioretinopathy. Retina. 2005;25:422-429.
- Cotticelli L, Borrelli M, D'Alessio AC, et al. Central serous chorioretinopathy and Helicobacter pylori. Eur J Ophthalmol. 2006;16:274-278.
- Tewari HK, Gadia R, Kumar D, et al. Sympathetic-parasympathetic activity and reactivity in central serous chorioretinopathy: a case-control study. Invest Ophthalmol Vis Sci. 2006;47:3474-3478.
- Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26–34.
- Jampol LM, Weinreb R, Yannuzzi L. Involvement of corticosteroids and catecholamines in the pathogenesis of central serous chorioretinopathy: a rationale for new treatment strategies. Ophthalmology. 2002;109:1765-1766
- Giovannini A, Scassellati-Sforzolini B, D'Altobrando E, et al. Choroidal findings in the course of idiopathic serous pigment epithelial detachment detected by indocyanine green videoangiography. Retina. 1997;17:286-293.
- Spitznas M. Pathogenesis of central serous retinopathy: A new working hypothesis. Graefes Arch Clin Exp Ophthalmol. 1986;224:321-324.
- Marmor MF. New hypotheses on the pathogenesis and treatment of serous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 1988;226:548-552.
- Guyer DR, Yannuzzi LA, Slakter JS, et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Opthalmol. 1994;112:1057-1062.
- Marmor M, Tan F. Central serous chorioretinopathy: bilateral multifocal electroretinographic abnormalities. Arch Opthalmol. 1999;117:184-188.
- Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103:2070-2080.
- Fackler, T, Bearelly, S, Odom, T, et al. Acute lymphoblastic leukemia presenting as bilateral serous macular detachments. Photo essay. Retina. 2006;26:710-712.
- Schmidt-Erfurth U, Schlötzer-Schrehard U, Cursiefen C, et al. Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2003;44:4473-4480.
- Colucciello M. Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina. 2006;26:239-242.
- Chan WM, Lai TY, Lai RY, et al. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina. 2008;28:85-93.
- Burumcek E, Mudun A, Karacorlu S, et al. Laser photocoagulation for persistent central serous retinopathy: results of long-term follow-up. Ophthalmology. 1997;104:1981-1982.
- Schatz H, Yannuzzi LA, Gitter KA. Subretinal neovascularization following argon laser photocoagulation treatment for central serous retinopathy: complication or misdiagnosis? Ophthalmology. 1977;83:893-906.
- Pikkel J, Beiran I, Ophir A, et al. Acetazolamide for central serous retinopathy. Ophthalmology. 2002;109:1723-1725.
- Tatham A, Macfarlane A. The use of propranolol to treat central serous chorioretinopathy: an evaluation by serial OCT. J Ocul Pharmacol Ther. 2006;22:145-149.
- Prunte C, Sommer M, Orgul S, Flammer J. Mixed alpha-beta-adrenergic antagonist treatment of central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 1998;39(Suppl):830.
- Nielsen, J; Weinreb, R; Yannuzzi, L, et al. Mifepristone treatment of chronic central serous chorioretinopathy. Retina. 2007;27:119-122.
- Meyerle CB, Freund KB, Bhatnagar P, et al. Ketoconazole in the treatment of chronic idiopathic central serous chorioretinopathy. Retina. 2007;27:943-946.
- Torres-Soriano ME, García-Aguirre G, Kon-Jara V, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (Case reports). Graefes Arch Clin Exp Ophthalmol. 2008 Jun 4; [Epub ahead of print].








