PEER REVIEWED
Intraocular Lens Implantation From the Vitreoretinal Perspective
ANNIE C. LEE, MD · SHARON FEKRAT, MD, FACS
With the exponential growth of intraocular lens (IOL) technologies that offer the anterior segment surgeon a multitude of options, it is important to consider how these IOLs affect the evaluation and management of eyes with vitreoretinal disease and eyes undergoing vitreoretinal surgery. This overview touches on various IOL features and their effect on issues of concern to the vitreoretinal surgeon, such as retinal visualization — both preoperatively and intraoperatively — posterior capsular opacification (PCO), and propensity for IOL dislocation, among others. We also review new and upcoming intraocular lens designs that may have implications for vitreoretinal management (see Table).
FEATURES OF THE INTRAOCULAR LENS
IOL Composition
Intraocular lens composition has changed over time. Polymethylmethacrylate (PMMA) has largely been replaced by foldable materials, most notably silicone and acrylic. Acrylic is currently the most popular IOL material and is available in hydrophilic and hydrophobic forms.
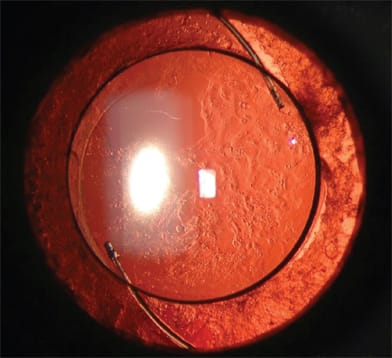
The development of PCO (Figure 1) varies with different IOL composition and interferes with the vitreoretinal examination preoperatively and intraoperatively. Even though an opacified posterior capsule can be remedied with a YAG laser or an intraoperative central capsulectomy, an open posterior capsule alters the way a vitreoretinal surgeon may approach and manage various scenarios during vitreoretinal surgery. PCO is most common with silicone IOLs.1-7 Silicone stimulates the production of collagen precursors and extracellular matrix by anterior subcapsular epithelial cells, leading to PCO. The rate of PCO is significantly lower with hydrophobic IOLs,8-12 which become coated with fibronectin, a naturally occurring bioadhesive, soon after implantation. This facilitates adherence to the capsule, isolating it from the immune system, resulting in less inflammation and opacification.
| Annie C. Lee, MD, is a resident at the Duke Eye Center in Durham, NC. Sharon Fekrat, MD, FACS, is associate professor of ophthalmology at Duke in the Vitreoretinal Surgical Center. Neither author reports any financial interest in any product mentioned in this article. Dr. Fekrat can be reached at fekra001@mc.duke.edu. |
ALL IMAGES APPEAR COURTESY OF MICHAEL P. KELLY, CPT, CHI, AND MEMBERS OF DUKE EYE IMAGING TEAM.
Figure 1. Posterior capsular opacification (PCO) highlighting Elschnig pearl formation following placement of multi-piece multifocal hydrophobic acrylic posterior chamber intraocular lens.
In addition to PCO, the IOL can opacify. The pathogenesis of IOL opacification is undetermined, with speculation that ultraviolet (UV) light is a contributory factor. Idiopathic brown opacification of IOLs (Figure 2) has also been reported.13-15 Glistening within some acrylic IOLs may be due to temperature-induced fluid formation in the optic related to Wagon Wheel and AcryPak packaging.16-19 Late opacification of hydrophilic IOLs can be particularly problematic.20-22
While no IOL, hydrophilic or hydrophobic, is immune to secondary calcification, hydrophilic acrylic IOLs easily accumulate calcified deposits when the concentrations of calcium, phosphate, and albumin in the aqueous humor increase following blood-aqueous barrier breakdown.23 Triamcinolone acetonide has a tendency to adhere and deposit onto hydrophilic acrylic IOLs in vitro and may obscure vitreoretinal visualization in implanted eyes.24 Hydrophilic acrylic IOLs have also demonstrated marked uptake of trypan blue, fluorescein sodium, and indocyanine green dyes used in the diagnosis and treatment of retinal disorders.25
Silicone oil droplets adhere to silicone IOLs and may affect visual acuity, a significant disadvantage of these IOLs in patients with complicated vitreoretinal pathology requiring use of intravitreal silicone oil.26,27 Also, when the posterior capsule is open, silicone IOLs develop intraoperative fogging from water vapor during air-fluid exchange more commonly than other types of IOLs, significantly reducing the surgeon's view during vitreoretinal surgery. Manual cleaning with a soft-tipped cannula and the application of viscoelastic are common intraoperative techniques to improve visualization, but the effect is suboptimal and transient.28-30 This intraoperative condensation is more easily removed from nonsilicone lenses.31
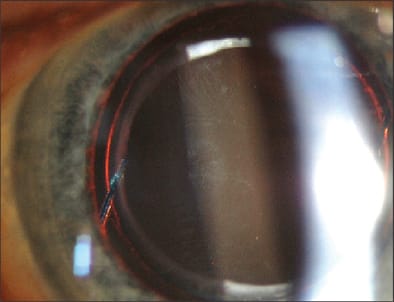
Figure 2. Capsular instability leading to marked superonasal decentration of a single-piece hydrophobic posterior chamber intraocular lens.
Optic Size
Most IOLs have an optic diameter of 6 mm. Smaller diameters may compromise an ophthalmologist's view of the vitreous and retina as well as interfere with treatment, particularly affecting stereopsis and visualization of the peripheral retina. Larger optic diameters (greater than 6 mm) facilitate the view of the vitreous and retina and have been associated with less PCO formation.32,33
Optic Edge Configuration
Increasing evidence suggests that optic edge configuration is more important than lens material in PCO pathogenesis. Rounded optic edges, which may reduce scotomas and dysphotopsias, are now less popular than sharp or square edges. The posterior square edge, forced against the capsule, may create an impediment to lens epithelial cell migration behind the optic. Multiple studies have reported a lower incidence of PCO with sharp-edge optics; however, the effect on anterior capsular opacification is less clear.34-37
IOL Construction
The configuration of the optic-haptic junction may contribute to IOL decentration (Figure 3), thus interfering with the examination and treatment of vitreoretinal diseases. Plate-haptic construction has largely fallen out of favor due to a higher rate of IOL dislocation, both spontaneously and following YAG laser capsulotomy.38-44 This higher rate of dislocation may be due to poor capsular fixation and increased posterior bowing. The silicone plate-haptic IOL is particularly susceptible to the forces of capsular contraction. Silicone does not adhere well to the capsule, and compression produces folding or buckling out of the plane of the IOL rather than compression within the plane, as with a loop-design IOL.45 Studies have not shown significant differences between single and multipiece IOLs with regards to tilt, decentration, or capsular contraction following implantation in an intact capsular bag.46,47
Position
Intraocular lenses have been designed for placement in various positions within the eye — in the capsular bag, in the sulcus, or in the anterior chamber. The IOL can also be iris-fixated or scleral-fixated. For scleral-fixated IOLs, the extent of both tilt and decentration after scleral suture fixation was greater than that observed after either out-of-the-bag or in-the-bag implantation.48
If capsular support is compromised during cataract surgery and there are no retained lens fragments, then selection of location of IOL placement is often dictated by the level of capsular support. If capsular support is insufficient, posterior IOL dislocation is more likely. If capsular support is compromised during cataract surgery and there are retained lens fragments, then most vitreoretinal surgeons prefer that the anterior segment surgeon insert an IOL prior to referral for vitrectomy and removal of the retained lens fragments. Again, location selection is dictated by the level of capsular support. If the entire nucleus has fallen into the vitreous cavity and is quite brunescent, then some vitreoretinal surgeons prefer that an IOL not be placed since it may be necessary to remove the hard nucleus through a corneoscleral wound.

Figure 3. Brown discoloration of a multipiece multifocal hydrophobic acrylic posterior chamber intraocular lens.

Table continued

During vitreoretinal surgery, an air-fluid exchange is sometimes performed. If there is an open posterior capsule, a decentered IOL, or an anterior chamber IOL, air frequently enters the anterior chamber and markedly hinders intraoperative viewing of the vitreous and retina. Gas and silicone oil are more likely to enter the anterior chamber intraoperatively and postoperatively in eyes with such IOL scenarios.
Light Filters
The implantation of yellow IOLs that absorb high-energy blue radiation may benefit patients with age-related macular degeneration by protecting the retina from these rays. These IOLs may also improve contrast sensitivity, although the data are inconclusive.49-51 The literature reports no intraoperative or functional disadvantage of these IOLs;52 however, anecdotal reports exist of decreased retinal illumination during surgery in implanted eyes. Alcon's (Fort Worth, TX) AcrySof Natural IOL is currently approved in the United States, and the photochromic Aurium lens (Medennium, Irvine, CA), which becomes yellow when exposed to UV light, is on the market in Europe.
DESIGN
Multifocal IOLs
Specialty presbyopia-correcting lenses have been gaining popularity among anterior segment surgeons and patients. Two multifocal IOLs were approved by the US Food and Drug Administration (FDA) in 2005. The ReStor (Alcon) and ReZoom (Advanced Medical Optics [AMO], Santa Ana, CA) IOLs are both composed of a hydrophobic acrylic material. The Tecnis Multifocal (AMO), under investigational study in the US, has been available in Europe in both silicone and hydrophobic acrylic forms. All multifocal models are designed with optics containing multiple concentric zones (Figure 4). While not yet formally reported, there are anecdotal reports of viewing disadvantages through a multizone optic when implanted eyes undergo vitreoretinal procedures. Wide-field viewing systems are reportedly helpful in combating this disadvantage.
Accommodative IOLs
Bausch & Lomb's (Rochester, NY) crystalens is the first accommodative IOL on the US market. The current model is a silicone biconvex lens with a single 5-mm optic and flexible hinged-plate haptics that allow forward movement of the optic during accommodative effort. Its smaller optic size may impair peripheral retinal examination, and silicone-related factors as discussed earlier are potential concerns.
Newer accommodative IOLs may incorporate dual-optic technology as with Visiogen's (Irvine, CA) Synchrony IOL, now in FDA phase 3 testing. A 5.5-mm high-powered anterior silicone optic is connected to a 6-mm negative-powered optic by haptics that act like springs. While the dual-optic design may potentially allow for greater accommodation, the bulkier apparatus may interfere with posterior segment viewing.

Figure 4. Concentric diffractive zones of a multifocal single-piece hydrophobic acrylic posterior chamber intraocular lens (ReStor, Alcon).
Another accommodative IOL under development is the thermodynamic Smart IOL (Medennium). This lens is composed of an injectable acrylic polymer with thermoplastic properties. A stable rod at room temperature, the Smart IOL reconfigures to a predetermined diopter power when inserted into the eye, completely filling the capsular bag. Because of its hydrophobic acrylic nature and the fact that it fills the capsular bag, PCO and decentration may be less likely. The long-term stability of the IOL in the eye is not yet known.
Phakic IOLs
Phakic IOLs may be offered to patients with high refractive errors. Two FDA-approved devices are currently available. The Verisyse (AMO) phakic IOL is a single-piece PMMA anterior chamber lens that is secured to the iris (Figure 5). Iris-fixation may limit pupillary dilation and subsequent vitreoretinal examination.53,54 In addition, all anterior chamber lenses can lead to poor visibility from corneal decompensation, a main indication for exchange.55 The Visian ICL or Implantable Collamer Lens (Staar, Monrovia, CA) is a single-piece phakic IOL that is positioned between the iris and crystalline lens. Precise sizing is difficult, and lenticular contact may lead to accelerated cataract formation, thus complicating vitreoretinal examination and surgical procedures.
Telescopic IOLs
The Implantable Miniature Telescope (IMT, VisionCare, Saratoga, CA), which is not FDA approved, has been studied in the visual rehabilitation of those with advanced age-related macular degeneration.56,57 The IMT is implanted in 1 eye to provide some central vision, while the nonimplanted eye provides peripheral vision for mobility and navigation. Long-term corneal health after implantation remains under investigation. The implantation of an IMT makes examination and treatment of the vitreous and retina more challenging and suboptimal, and the ophthalmologist's view through the IMT is greatly minified. Successful focal laser photocoagulation through an implanted device has been reported,58 but the safety of YAG laser capsulotomy in the event of PCO formation is not known.
The Lipshitz Macular Implant (LMI; OptoLight, Herzlia, Israel) has shown early success in India59 and has been reported to have fewer concerns about retinal view. The LMI is a traditional IOL incorporating 2 mirrors in Cassegrain telescopic configuration, magnifying the central image on the retina 2.5 times while preserving peripheral vision. The LMI may be implanted bilaterally. Authors of the study note that it was possible to view the central fundus from around the mirrors and that fluorescein angiography showed good visibility up to the midperiphery of the retina. Inadvertent reflection from the mirrors may lead to some glare on retinal examination, and ease of intraoperative visualization is not yet known.

Figure 5. Iris-fixation of a single-piece PMMA anterior chamber phakic intraocular lens (Verisyse, AMO).
Light Adjustable Lens
One of the most highly anticipated specialty lenses under investigation is the Light Adjustable Lens (LAL; Calhoun, Pasadena, CA). This silicone posterior chamber lens, once implanted in the eye, can be altered with laser light at 365 nm to adjust its power and toricity to the patient's satisfaction. In addition to silicone-related issues, the effect on the retina of multiple UV-light-mediated modifications may require further investigation.
SUMMARY
The choice of IOL by the anterior segment surgeon merits careful consideration, especially in patients with known vitreoretinal diseases and those who may require a vitreoretinal surgical procedure. The ideal IOL from the vitreoretinal perspective should have a large optic with sharp edge configuration, a design that does not impair vitreoretinal visualization, and a low likelihood of posterior capsular opacification, among other features. The implanted IOL should also be placed in a location that allows adequate pupillary dilation and offers good support to decrease the likelihood of posterior dislocation. If silicone oil is to be used, a non-silicone IOL is preferred. RP
REFERENCES
- Zambarakji HJ, Rauz S, Reynolds A, et al. Capsulorhexis phymosis following uncomplicated phacoemulsification surgery. Eye. 1997;11(Pt 5):635-638.
- Hayashi K, Hayashi H. Intraocular lens factors that may affect anterior capsule contraction. Ophthalmology. 2005;112:286-292.
- Park TK, Chung SK, Baek NH. Changes in the area of the anterior capsule opening after intraocular lens implantation. J Cataract Refract Surg. 2002;28:1613-1617.
- Hayashi H, Hayashi K, Nakao F, Hayashi F. Quantitative comparison of posterior capsule opacification after polymethylmethacrylate, silicone, and soft acrylic intraocular lens implantation. Arch Ophthalmol. 1998;116:1579-1582.
- Abhilakh Missier KA, Nuijts RM, Tjia KF. Posterior capsule opacification: silicone plate-haptic versus AcrySof intraocular lenses. J Cataract Refract Surg. 2003;29:1569-1574.
- Dahlhauser KF, Wroblewski KJ, Mader TH. Anterior capsule contraction with foldable silicone intraocular lenses. J Cataract Refract Surg. 1998;24:1216-1219.
- Luke C, Dietlein TS, Jacobi PC, et al. Massive anterior capsule shrinkage after plate-haptic silicone lens implantation in uveitis. J Cataract Refract Surg. 2001;27:333-336.
- Heatley CJ, Spalton DJ, Kumar A, et al. Comparison of posterior capsule opacification rates between hydrophilic and hydrophobic single-piece acrylic intraocular lenses. J Cataract Refract Surg. 2005;31:718-724.
- Javdani SM, Huygens MM, Callebaut F. Neodymium: YAG capsulotomy rates after phacoemulsification with hydrophobic and hydrophilic acrylic intraocular lenses. Bull Soc Belge Ophtalmol. 2002;13-17.
- Kugelberg M, Wejde G, Jayaram H, Zetterstrom C. Two-year follow-up of posterior capsule opacification after implantation of a hydrophilic or hydrophobic acrylic intraocular lens. Acta Ophthalmol Scand. 2007;
- Suh Y, Oh C, Kim HM. Comparison of the long-term clinical results of hydrophilic and hydrophobic acrylic intraocular lenses. Korean J Ophthalmol. 2005;19:29-33.
- Auffarth GU, Brezin A, Caporossi A, et al. Comparison of Nd : YAG capsulotomy rates following phacoemulsification with implantation of PMMA, silicone, or acrylic intra-ocular lenses in four European countries. Ophthalmic Epidemiol. 2004;11:319-329.
- Katai N, Yokoyama R, Yoshimura N. Progressive brown discoloration of silicone intraocular lenses after vitrectomy in a patient on amiodarone. J Cataract Refract Surg. 1999;25:451-452.
- Manuchehri K, Mohamed S, Cheung D, et al. Brown deposits in the optic of foldable intraocular lenses in patients with uveitis. Eye. 2004;18:54-58.
- Tanaka T, Saika S, Hashizume N, Ohnishi Y. Brown haze in an Allergan SI-40NB silicone intraocular lens. J Cataract Refract Surg. 2004;30:250-252.
- Gregori NZ, Spencer TS, Mamalis N, Olson RJ. In vitro comparison of glistening formation among hydrophobic acrylic intraocular lenses (1). J Cataract Refract Surg. 2002;28:1262-1268.
- Kato K, Nishida M, Yamane H, et al. Glistening formation in an AcrySof lens initiated by spinodal decomposition of the polymer network by temperature change. J Cataract Refract Surg. 2001;27:1493-1498.
- Omar O, Pirayesh A, Mamalis N, Olson RJ. In vitro analysis of AcrySof intraocular lens glistenings in AcryPak and Wagon Wheel packaging. J Cataract Refract Surg. 1998;24:107-113.
- Peetermans E, Hennekes R. Long-term results of wagon wheel packed acrylic intra-ocular lenses (AcrySof). Bull Soc Belge Ophtalmol. 1999;271:45-48.
- Neuhann IM, Kleinmann G, Apple DJ. A new classification of calcification of intraocular lenses. Ophthalmology. 2008;115:73-79.
- Tehrani M, Mamalis N, Wallin T, et al. Late postoperative opacification of MemoryLens hydrophilic acrylic intraocular lenses: case series and review. J Cataract Refract Surg. 2004;30:115-122.
- Oner HE, Durak I, Saatci OA. Late postoperative opacification of hydrophilic acrylic intraocular lenses. Ophthalmic Surg Lasers. 2002;33:304-308.
- Nakanome S, Watanabe H, Tanaka K, and Tochikubo T. Calcification of Hydroview H60M intraocular lenses: aqueous humor analysis and comparisons with other intraocular lens materials. J Cataract Refract Surg. 2008;34:80-86.
- Arikan G, Saatci AO, Sarioglu S, et al. Adherence of triamcinolone acetonide to various intraocular lens materials. J Cataract Refract Surg. 2005;31:1983-1985.
- Ozbek Z, Saatci AO, Durak I, et al. Staining of intraocular lenses with various dyes: a study of digital image analysis. Ophthalmologica. 2004;218:243-247.
- Bartz-Schmidt KU, Konen W, Esser P, et al. [Intraocular silicone lenses and silicone oil]. Klin Monatsbl Augenheilkd. 1995;207:162-166.
- Kusaka S, Kodama T, Ohashi Y. Condensation of silicone oil on the posterior surface of a silicone intraocular lens during vitrectomy. Am J Ophthalmol. 1996;121:574-575.
- Jaffe GJ. Management of condensation on a foldable acrylic intraocular lens after vitrectomy and fluid-air exchange. Am J Ophthalmol. 1997;124:692-693.
- Eaton AM, Jaffe GJ, McCuen BW, 2nd, Mincey GJ. Condensation on the posterior surface of silicone intraocular lenses during fluid-air exchange. Ophthalmology. 1995;102:733-736.
- Francese JE, Christ FR, Buchen SY, et al. Moisture droplet formation on the posterior surface of intraocular lenses during fluid/air exchange. J Cataract Refract Surg. 1995;21:685-689.
- Khawly JA, Lambert RJ, Jaffe GJ. Intraocular lens changes after short- and long-term exposure to intraocular silicone oil. An in vivo study. Ophthalmology. 1998;105:1227-1233.
- Meacock WR, Spalton DJ, Boyce JF, Jose RM. Effect of optic size on posterior capsule opacification: 5.5 mm versus 6.0 mm AcrySof intraocular lenses. J Cataract Refract Surg. 2001;27:1194-1198.
- Nishi O, Nishi K. Effect of the optic size of a single-piece acrylic intraocular lens on posterior capsule opacification. J Cataract Refract Surg. 2003;29:348-353.
- Buehl W, Findl O, Menapace R, et al. Effect of an acrylic intraocular lens with a sharp posterior optic edge on posterior capsule opacification. J Cataract Refract Surg. 2002;28:1105-1111.
- Abela-Formanek C, Amon M, Schild G, et al. Uveal and capsular biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses. J Cataract Refract Surg. 2002;28:50-61.
- Buehl W, Findl O, Menapace R, et al. Long-term effect of optic edge design in an acrylic intraocular lens on posterior capsule opacification. J Cataract Refract Surg. 2005;31:954-961.
- Hayashi K, Hayashi H. Posterior capsule opacification in the presence of an intraocular lens with a sharp versus rounded optic edge. Ophthalmology. 2005;112:1550-1556.
- Gonzalez GA, Irvine AR. Posterior dislocation of plate haptic silicone lenses. Arch Ophthalmol. 1996;114:775-776.
- Jayaram H, Goel R, Whitefield L. Zonular disinsertion five years after implantation of a plate haptic silicone intraocular lens. Eye.2005;19:480-482.
- Mackool RJ. Decentration of plate-haptic lenses. J Cataract Refract Surg. 1996;22:396.
- Akerele T, Minasian M, Little B, Jagger J. Posterior dislocation of Staar plate haptic silicone lenses following Nd:YAG capsulotomy. Eye. 1999;13(Pt 5):700-702.
- Petersen AM, Bluth LL, Campion M. Delayed posterior dislocation of silicone plate-haptic lenses after neodymium:yag capsulotomy. J Cataract Refract Surg. 2000;26:1827-1829.
- Tuft SJ, Talks SJ. Delayed dislocation of foldable plate-haptic silicone lenses after Nd:YAG laser anterior capsulotomy. Am J Ophthalmol. 1998;126:586-588.
- Faucher A, Rootman DS. Dislocation of a plate-haptic silicone intraocular lens into the anterior chamber. J Cataract Refract Surg. 2001;27:169-171.
- Carlson AN, Stewart WC, Tso PC. Intraocular lens complications requiring removal or exchange. Surv Ophthalmol. 1998;42:417-440.
- Nejima R, Miyai T, Kataoka Y, et al. Prospective intrapatient comparison of 6.0-millimeter optic single-piece and 3-piece hydrophobic acrylic foldable intraocular lenses. Ophthalmology. 2006;113:585-590.
- Mutlu FM, Erdurman C, Sobaci G, Bayraktar MZ. Comparison of tilt and decentration of 1-piece and 3-piece hydrophobic acrylic intraocular lenses. J Cataract Refract Surg. 2005;31:343-347.
- Hayashi K, Hayashi H, Nakao F, Hayashi F. Intraocular lens tilt and decentration, anterior chamber depth, and refractive error after trans-scleral suture fixation surgery. Ophthalmology. 1999;106:878-882.
- Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006;84:4-15.
- Braunstein RE, Sparrow JR. A blue-blocking intraocular lens should be used in cataract surgery. Arch Ophthalmol. 2005;123:547-549.
- Espindle D, Crawford B, Maxwell A, et al. Quality-of-life improvements in cataract patients with bilateral blue light-filtering intraocular lenses: clinical trial. J Cataract Refract Surg. 2005;31:1952-1959.
- Falkner-Radler CI, Benesch T, Binder S. Blue light-filter intraocular lenses in vitrectomy combined with cataract surgery: results of a randomized controlled clinical trial. Am J Ophthalmol. 2008;145:499-503.
- Lemarinel B, Racine L, Rohart C, et al. [Long-term changes in pupil size after implantation of an Artisan phakic intraocular lens for correction of high myopia]. J Fr Ophtalmol. 2007;30:11-16.
- McHugh D, Wong D, Chignell A, et al. Pseudophakic retinal detachment. Graefes Arch Clin Exp Ophthalmol. 1991;229:521-525.
- Auffarth GU, Wesendahl TA, Brown SJ, Apple DJ. [Complications after implantation of anterior chamber lenses. An analysis of 4,100 explanted intraocular lenses]. Ophthalmologe. 1994;91:512-517.
- Hudson HL, Lane SS, Heier JS, et al. Implantable miniature telescope for the treatment of visual acuity loss resulting from end-stage age-related macular degeneration: 1-year results. Ophthalmology. 2006;113:1987-2001.
- Lane SS, Kuppermann BD. The Implantable Miniature Telescope for macular degeneration. Curr Opin Ophthalmol. 2006;17:94-98.
- Garfinkel RA, Berinstein DM, Frantz R. Treatment of choroidal neovascularization through the implantable miniature telescope. Am J Ophthalmol. 2006;141:766-767.
- Agarwal A, Lipshitz I, Jacob S, et al. Mirror telescopic intraocular lens for agerelated macular degeneration: design and preliminary clinical results of the Lipshitz macular implant. J Cataract Refract Surg. 2008;34:87-94.








