PEER REVIEWED
Radiation Therapy for Neovascular Age-related Macular Degeneration
MEHRAN TABAN, MD · DARIUS MOSHFEGHI, MD · RISHI P. SINGH, MD
The past decade has brought about a dramatic upgrade in our management of neovascular age-related macular degeneration (AMD). The advent of photodynamic and anti-vascular endothelial growth factor (VEGF) therapies, in particular, has greatly improved the prognosis for preservation or even improvement of vision in this population.1,2 However, all these therapies have some drawbacks. Photodynamic therapy (PDT) reduces moderate vision loss only modestly, and only a few patients experience improved visual acuity (VA). Moreover, the recurrence rate is high, with >90% of patients requiring retreatment after 3 months.3 Ranibizumab (Lucentis, Genentech), perhaps the best proven treatment of wet AMD to date, requires multiple retreatments, as suggested by the PrONTO study, with the mean number of injections for the first year being 5.6.4 Intravitreal injections carry the risk of retinal tears, rhegmatogenous retinal detachment, vitreous hemorrhage, hypotony, endophthalmitis, and pseudoendophthalmitis.5,6 Furthermore, social and budgetary burdens of repeated visits, injection fees, and drug costs are large, and given the increasing number of AMD patients, alternative therapies with fewer retreatments would be ideal.
Thus, there still exists the need for the optimum treatment modality that combines the best aspects from all these therapies, namely, improvement and stabilization of vision, few side effects (ocular or systemic), fewer retreatments, and low costs. One such potential treatment is radiation therapy. This review aims to provide a synopsis of the history and rationale of radiation therapy as an adjunctive agent for the management of AMD, as well as summarize its most important randomized trials and future directions.
| Mehran Taban, MD, is a retinal physician at the Cole Eye Institute of the Cleveland Clinic. Darius Moshfeghi, MD, is director of pediatric vitreoretinal surgery and the director of ocular oncology in the Department of Ophthalmology, Stanford University School of Medicine in Stanford, CA. Rishi P. Singh, MD, is on the associate staff of the Cole Eye Institute. Dr. Taban reports no financial interest in any companies mentioned in this article. Dr. Moshfeghi reports minimal financial interest in Clarity Medical Systems, Compare Networks, Medavec, Genentech, and InSitu Therapeutic, and moderate financial interest in Oraya. Dr. Singh reports minimal financial interest in Oraya. Dr. Singh can be reached via e-mail at singhr@ccf.org. |
HISTORICAL PERSPECTIVE
Ionizing radiation can be categorized by the means of its delivery. In brachytherapy, the ionizing radiation source is delivered directly to the lesion via surgery or other intervention. The source used in brachytherapy is traditionally an isotope, which produces ionizing radiation in the form of beta particles as it decays. The particles act over a very short distance, which is why brachytherapy is delivered directly to the pathology of interest. Teletherapy or remote irradiation is the term given to radiation formed into a beam, which can be projected at an internal body target from an external source. External beam radiation (EBR) is the more modern term given to this type of therapy, which delivers ionizing radiation electronically. Proton beam is another method for the delivery of radiation, and it allows a higher dose to be delivered to a specific area. Like most forms of EBR, proton-beam therapy requires an entry site and irradiates within its path. The dose volume is limited to a section of the eye, with decreasing irradiation of normal tissues outside the beam and the fellow eye. Stereotactic radiotherapy or stereotactic radiosurgery is the term given to teletherapy devices that direct beams from different angles relative to the target area, so as to minimize exposure to surrounding healthy tissues and precisely localize energy delivery.
Although radiation therapy was used to treat retinal neovascularization as early as 1948 and possibly as early as 1919,7 it was not until 1993, when Chakravarthy et al.8 in Northern Ireland published their data, that the era of radiation therapy for neovascular AMD began. Since then, a multitude of studies using various doses and fractions have been published. Some have shown better maintenance of VA,9-13 while others failed to show any benefit.14-20
In animal studies, low-dose radiation applied focally at the site of an ocular perforation causes a marked reduction in the vascularity of the granulation tissue.21 Focal radiation to doses of 16 Gy did not adversely affect the adjacent healthy retina and choroid, based on histologic evaluation.21 Based on this information, Chakravarthy et al. piloted a study of the treatment of age-related neovascular membranes by teletherapy.8 In this study, 19 of 26 patients (7 controls) were treated using 10 to 15 Gy in divided doses. At 1 year, 63% of treated patients showed stabilization of vision, whereas there was deterioration of VA in all control eyes. Furthermore, significant neovascular membrane regression was recorded in 77% of treated patients, with concurrent progressive enlargement of the neovascular membrane in all controls.8
RATIONALE FOR RADIATION THERAPY
The use of low-dose radiation therapy for neovascular growth is based on experimental and clinical evidence and does have a sound scientific basis. Radiation has the ability to destroy vascular tissue, and low-dose radiation has been shown to inhibit new blood-vessel growth.22-24 This inhibition was first noted in plaque-irradiated choroidal melanomas. After plaque removal, a ring of chorioretinal atrophy is evident around the tumor's base, and fluorescein angiography shows decreased or absent blood flow in this area.25 However, in the treatment of macular degeneration, chorioretinal atrophy is an unacceptable endpoint.
Theoretically, precise radiation delivery to the macula can selectively inhibit proliferating endothelial cells, with limited destruction of retinal tissue and no systemic side effects. Moreover, Takahashi and colleagues found that new capillaries or vessels are more sensitive than larger vessels or fibroblasts.26 Vascular endothelial cells in particular are more radiosensitive than other mesenchymal cells types, such as fibroblasts and smooth muscle cells.27 As an additional benefit, radiation inhibits the inflammatory response, which is thought by many to play a role in the formation of choroidal neovascularization (CNV). This is particularly useful, since inflammatory cells and macrophages are found in choroidal-neovascular complexes.28 Because macrophages are known to release proangiogenic cytokines and growth factors, effectively shutting these vessels down would lead to less CNV recurrence and decreased need for retreatment.
The mechanism of capillary closure is threefold. First, radiation causes swelling of endothelial cytoplasm, which causes mechanical obstruction and downstream damage from the relatively anoxic conditions. Second, radiation causes the release of vasoactive substances, leading to vessel thrombosis and closure. Finally, cytokine release results in inflammation and additional tissue damage and closure.
Animal studies have validated that the use of EBR has little effect on normal retinal tissues. In rats, photoreceptor cell death is not seen at doses less than 10 Gy, and the retinal pigment epithelial cell loss does not occur under 20 Gy in single fractions. Histopathological analysis of patients treated with EBR for exudative AMD has been performed in a variety of studies.29,30 None of the studies has shown photoreceptor loss or damage with the development of fibrovascular changes within the choroidal-neovascular membrane.
As further support for the use of radiation to promote neovascular regression, other abnormal blood-vessel formations, including benign intracerebral arteriovenous malformations and choroidal hemangiomas, have been shown to regress with the use of ionizing beams.31,32
| Features of Radiation Retinopathy |
|---|
|
The purpose of radiotherapy in neovascular AMD would be multifold. First, it would attenuate the acute and delayed inflammatory response and subsequent CNV reactivation. Second, it would inhibit the rapid formation of fibroblasts after treatment and thus lead to less scar formation (as it does in the treatment of dermal keloids). Finally, it would lead to the closure of rapidly dividing endothelial cells, which is the main pathological component of CNV.
With any potential treatment, there is always risk for side effects. The potential toxicity of radiation is well known.22,25 Controllable factors that influence the development of radiation retinopathy include total dose delivered, daily fraction size, pre-existing microangiopathy, diabetes, and prior chemotherapy.22,25 Radiation-induced retinopathy has been reported at doses of 30 Gy to 35 Gy, but it is more commonly associated with doses of 45 Gy to 60 Gy. Radiation optic neuropathy is rare at doses lower than 50 Gy.22,25
CLINICAL STUDIES UTILIZING RADIATION FOR NEOVASCULAR AMD
Radiotherapy for AMD was pioneered by Chakravarthy in 1993.8 Since then, there have been a number of pilot studies (from small case series to relatively large randomized multicenter trials) to examine the potential of radiation therapy (10-50 Gy) for CNV due to AMD with variable results, some showing benefit,9-13 and others failing to show any significant benefit over observation.14-20
A major review of randomized controlled studies of radiotherapy for AMD was published by the Cochrane Collaboration in 2004.33 They included 1078 patients in 11 trials (range of 27 to 205 patients per trial) of EBR radiation with dosage ranging from 7.5 Gy to 24 Gy.9-11,13-16,34-37 The review found that "most trials showed effects (not always significant) that favored treatment with radiotherapy but with inconsistencies in the results."33 However, it should be noted that this review was for EBR trials up to 2004 and does not include more recent ongoing trials or different modalities, such as NeoVista (Fremont, CA). With regard to adverse effects, the incidence was low in all the trials reviewed. There were no reported cases of radiation retinopathy, optic neuropathy, or development of malignancy among the reviewed trials. However, the duration of follow-up was likely to be too short to detect all of these accurately.
Major Randomized Controlled Trials
The first randomized, controlled clinical trial was published by Bergink et al. in 1998.9 Their series, which included 74 patients with subfoveal CNV of any type, showed that, in patients treated with 24 Gy (fractions of 6 Gy), preservation of vision was significantly better in the treatment group compared with the observation group at 12 months, as moderate vision loss (>3 lines) occurred in 32% and 52%, respectively, in each group. The size of the CNV membranes, however, did not show any statistically significant difference between the treated group and untreated group.9
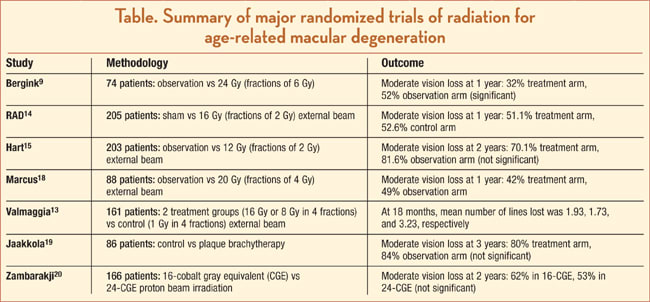
The largest randomized trial of radiation therapy for AMD was conducted in Germany in 1999.14 The Radiation Therapy for Age-Related Macular Degeneration (RAD) study was a multicenter, randomized, double-masked trial of 205 patients with subfoveal CNV (classic or occult lesions less than 6 Macular Photocoagulation Study disc areas). Patients were randomized to either 8 fractions of 2 Gy EBR or to control sham therapy.14 At 1 year, no significant benefit to radiation therapy was noted, as moderate vision loss (>3 lines) occurred in 51.1% of the treatment group and 52.6% of the control group. The unfortunate inclusion of patients with occult lesions, which by natural history are more indolent than classic lesions, may be a reason for these inconclusive results.14
The second largest randomized trial of radiation therapy for AMD is the Subfoveal Radiotherapy Study (SFRADS).15 Two hundred three subjects with subfoveal CNV of any type were randomized to either EBR of 12 Gy in 6 fractions or observation.15 At 2 years, no significant benefit to radiation therapy was noted as moderate vision loss (>3 lines) occurred in 70.1% of the treatment group and 81.6% of the control group.15
The Age-Related Macular Degeneration Radiotherapy Trial (AMDRT) similarly reported no benefit from EBR in this multicenter, randomized, controlled trial of 88 patients with any type of subfoveal CNV.18 Subjects were randomized to either external beam radiation (20 Gy in 5 fractions) or observation (sham radiation). At 1 year, moderate vision loss (>3 lines) occurred in 42% of the treatment group and 49% of the control group.18
Valmaggia et al. reported favorable effects of EBR in their randomized trial in Switzerland.13 One hundred sixty-one patients with subfoveal CNV of any type were randomized to 2 treatment groups (16 Gy vs 8 Gy in 4 fractions) or control group (1 Gy in 4 fractions). At 18 months, the mean number of lines lost in VA were 1.93, 1.73, and 3.23, respectively.13
A similar Japanese study also reported favorable effect with EBR in a randomized, prospective, placebo-controlled trial of patients with subfoveal CNV of any type with a 2-year follow-up.10 They also reported no significant treatment-related side effects, using a total dose of 20 Gy delivered in 10 divided doses over 14 days. They concluded that radiotherapy showed a beneficial effect compared with untreated eyes and identified favorable factors, such as smaller area of CNV and better initial VA.10
Plaque brachytherapy with strontium 90 was evaluated in a randomized controlled trial of 86 patients in Finland.19 Radiotherapy was given via an episcleral approach with 2 different plaque sizes and durations. At 3 years, moderate vision loss (>3 lines) occurred in 80% of the treatment group and 84% of the control group, which was not statistically significant.19
Zambarakji and colleagues from Boston recently published their outcomes of proton-beam irradiation for neovascular AMD.20 One hundred sixty-six patients were randomized to either 16 cobalt gray equivalent (CGE) or 24 CGE proton radiation in 2 equal fractions. At 2 years, moderate vision loss (>3 lines) occurred in 62% in the 16-CGE group and 53% in the 24-CGE group (P=.40). However, there was no placebo or sham control group in this study.20
Collectively, these trials have led to equivocal or less effective therapy than current market agents. Why have these trials failed to show statistically significant efficacy outcomes? As depicted in the Figure by Marcus et al., the linear accelerator, such as that typically used in the above-mentioned studies, projects a wide beam across ipsilateral and contralateral critical structures, with 63% of the dose being delivered to the contralateral brain, 1.5% to the ipsilateral optic nerve, and 1.2% to the ipsilateral lens.16 Due to this imprecision, only a small amount of radiation was applied at each setting.

Figure. Radiation distribution using a linear accelerator for AMD therapy.
Therefore, the previous external beam studies have a major drawback: The radiation delivered to the macula was limited by technical inability to precisely localize energy on target. Consequently, small doses were given over a longer period of time (fractionation). No data support the assertion that radiation fractions are indeed cumulative in biologic effect on CNV (ie, that 8 doses of 2 Gy are equivalent to a single 16 Gy dose). Furthermore, the patients' eyes were not mapped in space relative to the X-ray beam and were not immobilized throughout treatment. Thus, confirmation and stability of beam targeting during the therapeutic fraction were not achieved, and fixed-beam, high-energy devices directed radiation across critical structures, such as the lens, the ciliary bodies, and the optic nerve.
FUTURE DIRECTIONS
Early NeoVista Results
NeoVista uses strontium-90 beta radiation. The device delivers radiation (24 Gy, single fraction) to the fovea via a strontium-90-tipped probe, inserted epiretinally after a partial vitrectomy. Early feasibility studies, presented at the 2007 American Academy of Ophthalmology meeting, for a cohort of subjects with any type of subfoveal CNV due to AMD suggest effectiveness. The initial study (N=34), delivering strontium-90 beta radiation alone, is now maturing and the cohort has reached month 18 follow-up. Data are indicating that the delivery procedure is safe and that, at month 18, 94% of subjects have lost <15 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters, and 24% have achieved >3 lines of vision improvement with a mean vision gain of 5.9 ETDRS letters with only 1 treatment. A second feasibility study (N=34) combines strontium-90 beta radiation with concomitant injections of bevacizumab (Avastin, Genentech). Data from this study have matured to 1-year follow-up, and the results are quiet encouraging. At month 12, 96% of subjects have lost <15 ETDRS letters and 48% have achieved >3 lines of vision improvement, with a mean vision gain of 13.1 ETDRS letters. Of note, only 5 subjects (15%) required more than 1 bevacizumab injection (4 subjects received 2 total injections, and 1 subject received 3 total injections).
NeoVista is currently involved in pivotal phase 3 trial comparing the effects of epiretinal strontium-90 beta radiation combined with ranibizumab to ranibizumab alone. This study, CABERNET, is a prospective, randomized, controlled, noninferiority study. The investigational arm consists of a 1-time treatment of strontium 90 delivered concomitantly with 2 ranibizumab injections — one at the time of radiation delivery and the second occurring at month 1. The control arm receives the PIER treatment regimen of ranibizumab with a major difference: Subjects in both arms are evaluated monthly and are eligible for rescue therapy according to protocol criteria. Durability of ≥24 months is expected with single treatment of radiation.
Unfortunately, the drawbacks to the procedure include the need for core vitrectomy for every patient, with the risks of cataract and vitreoretinal surgery. Furthermore, the treatment period requires the operator to hold the probe at a fixed distance from the macula for 4 to 5 minutes, likely resulting in an imprecise delivery if the probe is brought too close to the retinal surface or too close to the optic nerve.
Further Trials
Oraya Therapeutics (Newark, CA) is developing the first EBR system designed and built specifically for the eye. Among the many potential applications for ocular disorders, wet AMD will be the first application. The design incorporates eye tracking, lesion targeting through coupling to optical coherence tomography and A-scan ultrasound, and gating. Furthermore, the device can be placed in an ophthalmology clinic like a laser. Clinical technical performance data will be available soon.
Another company, Theragenics (Buford, GA), is in planning stages to utilize palladium-103 radiation, delivered through a trans-scleral device. This is in contrast to palladium-103 radiotherapy via plaque (brachytherapy), which has been evaluated in nonrandomized studies.38,39
SUMMARY
During the past decade, there have been a multitude of published studies on radiation therapy for neovascular AMD. While some have not shown any significant benefit, others have illustrated a potential future for this therapy. Recent preliminary results of the NeoVista Epi-Rad90 technology have begun to underscore the potential synergistic effects of ionizing radiation and anti-VEGF agents, both of which target rapidly proliferating cells. The advantages of combining these therapies have already been described in oncologic literature.40 RP
REFERENCES
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419-1431.
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432-1444.
- Verteporfin In Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: Two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization–verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131:541-560.
- Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomographyguided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566-583.
- Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intravitreous injection: a comprehensive review. Retina. 2004;24:676-698.
- Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003;136:791-796.
- Guyton JS, Reese AB. Use of Roentgen therapy for retinal diseases characterized by new-formed blood vessels. Arch Ophthalmol. 1948;40:389-412.
- Chakravarthy U, Houston RF, Archer DB. Treatment of age-related subfoveal neovascular membranes by teletherapy: A pilot study. Br J Ophthalmol. 1993;77:265-273.
- Bergink GJ, Hoyng CB, van der Maazen RW, Vingerling JR, van Daal WA, Deutman AF. A randomized controlled clinical trial on the efficacy of radiation therapy in the control of subfoveal choroidal neovascularization in age-related macular degeneration: Radiation versus observation. Graefes Arch Clin Exp Ophthalmol. 1998;236:321-325.
- Kobayashi H, Kobayashi K. Age-related macular degeneration: Long-term results of radiotherapy for subfoveal neovascular membranes. Am J Ophthalmol. 2000;130:617-635.
- Char DH, Irvine AI, Posner MD, Quivey J, Phillips TL, Kroll S. Randomized trial of radiation for age-related macular degeneration. Am J Ophthalmol. 1999;127:574-578.
- Berson AM, Finger PT, Sherr DL, Emery R, Alfieri AA, Bosworth JL. Radiotherapy for age-related macular degeneration: preliminary results of a potentially new treatment. Int J Radiat Oncol Biol Phys. 1996;36:861-865.
- Valmaggia C, Ries G, Ballinari P. Radiotherapy for subfoveal choroidal neovascularization in age-related macular degeneration: A randomized clinical trial. Am J Ophthalmol. 2002;133:521-529.
- Radiation Therapy for Age-Related Macular Degeneration (RAD) Study Group. A prospective, randomized, double-masked trial on radiation therapy for neovascular age-related macular degeneration (RAD study.. radiation therapy for age-related macular degeneration. Ophthalmology. 1999;106:2239-2247.
- Hart PM, Chakravarthy U, Mackenzie G, et al. Visual outcomes in the subfoveal radiotherapy study: A randomized controlled trial of teletherapy for age-related macular degeneration. Arch Ophthalmol. 2002;120:1029-1038.
- Marcus DM, Sheils W, Johnson MH, et al. External beam irradiation of subfoveal choroidal neovascularization complicating age-related macular degeneration: one-year results of a prospective, double-masked, randomized clinical trial. Arch Ophthalmol. 2001;119:171-180.
- Stalmans P, Leys A, Van Limbergen E. External beam radiotherapy (20 Gy, 2 Gy fractions) fails to control the growth of choroidal neovascularization in age-related macular degeneration: a review of 111 cases. Retina. 1997;17:481-492.
- Marcus DM, Peskin E, Maguire M, et al. The age-related macular degeneration radiotherapy trial (AMDRT): One year results from a pilot study. Am J Ophthalmol. 2004;138:818-828.
- Jaakkola A, Heikkonen J, Tommila P, Laatikainen L, Immonen I. Strontium plaque brachytherapy for exudative age-related macular degeneration: three-year results of a randomized study. Ophthalmology. 2005;112:567-573.
- Zambarakji HJ, Lane AM, Ezra E, et al. Proton beam irradiation for neovascular age-related macular degeneration. Ophthalmology. 2006;113:2012-2019.
- Chakravarthy U, Biggart JH, Gardiner TA, Archer DB, Maguire CJ. Focal irradiation of perforating eye injuries. Curr Eye Res. 1989;8:1241-1250.
- Flaxel CJ. Use of radiation in the treatment of age-related macular degeneration. Ophthalmol Clin North Am. 2002;15:437-444.
- Krishnan L, Krishnan EC, Jewell WR. Immediate effect of irradiation on microvasculature. J Radiat Oncol Biol Phys. 1988;15:147-150.
- Finger PT, Chakravarthy U, Augsburger JJ. Radiotherapy and the treatment of age-related macular degeneration. External beam radiation therapy is effective in the treatment of age-related macular degeneration. Arch Ophthalmol. 1998;116:1507-1511.
- Finger PT. Radiation therapy for choroidal melanoma. Surv Ophthalmol. 1997;42:215-232.
- Takahashi Y, Teshima T, Kawaguchi N, et al. Heavy ion irradiation inhibits in vitro angiogenesis even at sublethal dose. Cancer Res. 2003;63:4253-4257.
- Johnson LK, Longenecker JP, Fajardo LF. Differential radiation response of cultured endothelial cells and smooth myocytes. Anal Quant Cytol. 1982;4:188-198.
- Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37:855-868.
- Aisenbrey S, Lafaut BA, Reynders S, et al. Clinicopathological correlation of choroidal neovascularization after external beam radiotherapy in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2003;241:269-276.
- Lambooij AC, Kuijpers RW, Mooy CM, Kliffen M. Radiotherapy of exudative age-related macular degeneration; a clinical and pathologic study. Graefes Arch Clin Exp Ophthalmol. 2001;239:539-543.
- Engenhart R, Wowra B, Debus J, et al. The role of high-dose, single-fraction irradiation in small and large intracranial arteriovenous malformations. Int J Radiat Oncol Biol Phys. 1994;30:521-529.
- Schilling H, Sauerwein W, Lommatzsch A, et al. Long-term results after low dose ocular irradiation for choroidal haemangiomas. Br J Ophthalmol. 1997;81:267-273.
- Sivagnanavel V, Evans JR, Ockrim Z, Chong V. Radiotherapy for neovascular age-related macular deneration. Cochrane Database Syst Rev. 2004;4:CD004004.
- Ciulla TA, Danis RP, Klein SB, et al. Proton therapy for exudative age-related macular degeneration: A randomized, sham-controlled clinical trial. Am J Ophthalmol. 2002;134:905-906.
- Anders N, Stahl H, Dorn A, et al. Radiotherapy of exudative senile macular degeneration. A prospective controlled study. Ophthalmologe. 1998;95:760-764.
- Eter N, Schüller H, Spitznas M. Radiotherapy for age-related macular degeneration: is there a benefit for classic CNV? Int Ophthalmol. 2001;24:13-19.
- Kacperek A, Briggs M, Sheen M, Damato BE, Errington RD, Harding S. Macular degeneration treatment at Clatterbridge Centre for oncology: treatment and preliminary results. Physica Medica. 2001;17(Suppl 3):7-9.
- Finger PT, Gelman YP, Berson AM, Szechter A. Palladium-103 plaque radiation therapy for macular degeneration: results of a 7 year study. Br J Ophthalmol. 2003;87:1497-1503.
- Finger PT, Berson A, Sherr D, Riley R, Balkin RA, Bosworth JL. Radiation therapy for subretinal neovascularization. Ophthalmology. 1996;103:878-89.
- Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145-147.








