Retinal and Uveal Drug Toxicity
ESTHER KIM, BA · HOWARD F. FINE, MD, MHSC · MICHAEL D. OBER, MD
There are a number of medications that can be toxic to the eye. While the adverse effects of many of these are reversible upon drug cessation if detected early enough, others cause irreversible harm. Retinal physicians must employ their clinical acumen, as well as a host of imaging technologies including fluorescein angiography (FA), optical coherence tomography (OCT), ultrasonography, perimetry, electroretinography (ERG), and even fundus autofluorescence (FAF), to detect the earliest signs of retinal and uveal drug toxicity. This review discusses the major classes and prototypical drugs that cause retinal and uveal toxicity, organized by the anatomic locations they affect.
CRYSTALLINE RETINOPATHY: TAMOXIFEN, CANTHAXANTHINE, METHOXYFLURANE
Tamoxifen
Tamoxifen is a first-generation selective estrogen receptor modulator that has revolutionized the management of breast cancer. Currently, it is used to treat early and advanced stages of estrogen-receptor–positive breast cancer in pre- and postmenopausal women.1 Intraretinal crystalline deposits, corneal opacities, macular edema, and focal fundus pigmentary changes, along with decreased visual acuity (VA), have been linked with tamoxifen doses greater than 60 mg/m2 per day.2 The macular toxicity of tamoxifen may also predispose to macular hole formation.3
Upon clinical examination, crystalline deposits appear to be located in the inner retina, most commonly within the parafoveal area (Figure 1). FA reveals foveolar hyperfluorescence, which was initially thought to represent cystoid macular edema (CME). However, more recent OCT data reveal thinning of the retina with disruption of the outer photoreceptor reflex and a lamellar cyst within the fovea.4,5 Histologically, intraretinal lesions from pathologic specimens stain positively for glycosaminoglycans.6 Electron microscopy shows small intracellular (3 to 10 μm) and large extracellular (30 to 35 μm) deposits in the macular and paramacular areas, respectively, that appear within axons representing products of axonal degeneration.7
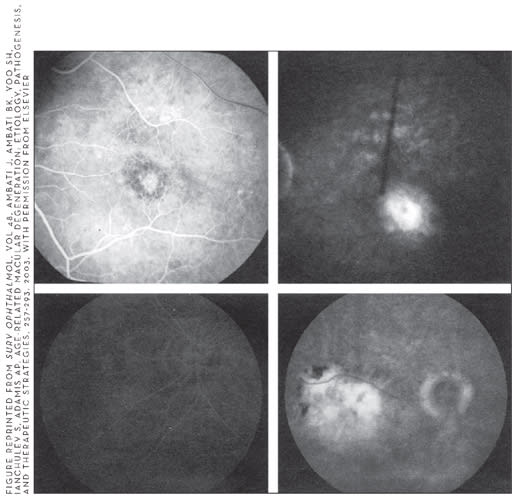
FIGURE 1 APPEARS COURTESY OF DRS. STANLEY CHANG AND HOWARD FINE.
Figure 1. Red-free fundus photograph of patient with tamoxifen retinopathy. Note the perifoveal crystals and peripapillary mottling of the retinal pigment epithelium.
| Esther Kim, BA, is a medical student at the New York University School of Medicine. Howard F. Fine, MD, MHSc, is the medical director of the Gerstner Clinical Research Center and assistant professor in the Department of Ophthalmology at Columbia University Medical Center in New York. Michael D. Ober, MD, practices ophthalmology in the Henry Ford Health System in Detroit. The authors have no financial interests to report. Dr. Fine can be reached at (212) 305-9355. |
Recent studies have shown ocular toxicity with the use of chronic low doses of tamoxifen as small as 20 mg/day and reports of intraretinal crystal formation even in asymptomatic patients.8,9 The frequency of ocular toxicity with long-term low-dose tamoxifen use is rare,10 generating controversy over the requirements for regular ophthalmic screenings in patients using low-dose tamoxifen. A study by Heier et al. looking at 135 asymptomatic patients found only 2 patients with characteristic retinal changes and concluded that special ophthalmic screening is not required when low doses of tamoxifen are prescribed, due to the rarity of ocular toxicity in these patients.11 Discontinuation of tamoxifen usually improves vision and edema but has no effect on the crystalline deposits.
Canthaxanthine
Canthaxanthine is a carotenoid related to beta-carotene, the substance responsible for producing the red/orange pigment in carrots and crustaceans, and was first isolated in edible Cantharellus mushrooms. It has been used therapeutically for vitiligo and photosensitivity disorders such as psoriasis and photosensitive eczema. Canthaxanthine is found in high doses in some over-the-counter oral tanning agents such as Orobronze, the use of which has been discontinued owing to toxicity. High-dose ingestion of canthaxanthine — at least 0.5 mg/kg/day12 — can produce the characteristic ring-shaped distribution of yellow-orange crystals surrounding the macula without associated vision loss. Clinically, patients are usually asymptomatic with no abnormal findings on FA. Upon examination, birefringent lipid-soluble crystals can be seen only in the macula, although they exist throughout the inner layers of the entire retina and ciliary body, predominantly around the fovea.13 A study of a primate model demonstrated that the presence of these crystals did not interfere with morphology or retinal function.14 ERG, electro-oculography (EOG), static perimetry, and dark adaptation findings can be abnormal but usually normalize months after stopping the drug.15-18 Light and transmission electron microscopic examination of cats following ingestion of canthaxanthine revealed changes in the retinal pigment epithelium (RPE) that included an increase in cell height and regional vacuolization due to an enlargement and disruption of phagolysosomes.19 After the discontinuation of canthaxanthine, the crystals may take several years to disappear clinically.20,21
FIGURE 2 REPRINTED FROM BULLOCK JD, ALBERT DM, SKINNER HC, MILLER WH, GALLA JH. CALCIUM OXALATE RETINOPATHY ASSOCIATED WITH GENERALIZED OXALOSIS : X-RAY DIFFRACTION AND ELECTRON MICROSCOPIC STUDIES OF CRYSTAL DEPOSITS. INVEST OPHTHALMOL. 197413:256-265. COURTESY OF INVESTIGATIVE OPHTHALMOLOGY AND V I SUAL SCIENCE.
Figure 2. H&E stain showing birefringent crystalline deposits in the RPE cell layer of the right eye using (A) low power, partially polarized light (× 25) and (B) partially polarized light (× 100).

FIGURE 3 APPEARS COURTESY OF OBER MD, EANDI C, FINE HF, YANNUZZI LA. CHAPTER 20 : MISCELLANEOUS MACULAR CONDITIONS, IN MACULAR SURGERY, 2ND ED, SLACK, IN PRESS.
Figure 3. FA images of the (A) mid right eye and (C) left eye and late frames (B) right eye and (D) left eye showing slight hyperfluorescence in the fovea despite marked CME and decreased visual acuity to 20/40.
Methoxyflurane
Methoxyflurane is an inhaled anesthetic and recognized nephrotoxin no longer used due to its pathological effects on the kidneys. Calcium oxalate crystals deposit in the kidneys and cause renal failure that may be irreversible in individuals with pre-existing renal insufficiency. Clinically, the patient can present with changes in the fundus consistent with a "flecked retina syndrome," which describe different fundus disorders characterized by numerous yellow-white spots varying in size and pattern with absence of vascular or optic nerve abnormalities.22 Funduscopic examination reveals multiple calcium oxalate crystals appearing as bright yellow-white punctuate lesions in the posterior pole and mid-periphery.23 Histologically, the crystals are also found in the RPE, neural retina, and ciliary epithelium and along the retinal arteries and arterioles (Figure 2).24,25
CYSTOID MACULAR EDEMA: NICOTINIC ACID, EPINEPHRINE, LATANOPROST
Nicotinic Acid
High doses of niacin were commonly used in the treatment of hyperlipidemia and hypercholesterolemia due to its ability to reduce serum lipid and cholesterol levels. However, its use has largely diminished since the introduction of better tolerated and more effective "statins," or HMG-CoA reductase inhibitors. There have been recent reports of high-dose niacin use in individuals attempting to defeat urine drug tests, especially for drugs such as marijuana.26 Although skin flushing is the most common side effect of niacin toxicity, macular edema that is silent on FA is a rare but well-known reversible ocular side effect that may occur with extremely high doses of niacin.27 Patients experience blurred vision that is sometimes associated with eyelid edema, sicca-like syndrome, paracentral scotoma, or metamorphopsia.28 Clinical examination reveals cystoid spaces within the fovea with no evidence of vascular leakage on FA (Figure 3).29 OCT confirms cystic retinal edema that is indistinguishable from other etiologies of edema (Figure 4).30,31 This has led to speculation that niacin-induced macular edema is a consequence of a direct toxic effect on Müller cells rather than a vasculopathy.32 Discontinuation of niacin usually restores vision with no long-term visual sequelae.

FIGURE 4 APPEARS COURTESY OF OBER MD, EANDI C, FINE HF, YANNUZZI LA. CHAPTER 20: MISCELLANEOUS MACULAR CONDITIONS, IN MACULAR SURGERY, 2ND ED, SLACK , IN PRESS.
Figure 4. Red-free photographs of the (A) right and (C) left eye and OCT of the (B) right and (D) left eyes of the patient demonstrating CME during acute niacin toxicity.
Epinephrine/Dipivefrin
Epinephrine and its prodrug dipivefrin had been widely used in topical formulations for the treatment of glaucoma before more effective ocular antihypertensives were introduced to the market. When applied topically, epinephrine and dipivefrin act to lower intraocular pressure (IOP) by increasing outflow and decreasing production of aqueous humor; conversely, when epinephrine is administered systemically, such as for anaphylactic shock, it can induce pupillary block and secondary angle-closure glaucoma.33 Maculopathy due to the use of topical epinephrine or dipivefrin in aphakic patients has been well documented. Patients usually complain of transient blurred vision several days to months after the initiation of therapy, which can be accompanied by decreased VA and CME.34 Discontinuation of the offending agent typically leads to the recovery of VA and resolution of CME, which can be followed by FA and/or OCT.35 Therefore, topical epinephrine should be avoided for treating glaucoma in aphakic and pseudophakic patients.
LATANOPROST
Latanoprost (Xalatan, Pfizer) is a prostaglandin analogue used topically in patients with glaucoma and ocular hypertension. It contributes to collagen structural changes in the ciliary muscle that result in enhancement of uveoscleral outflow, thereby decreasing IOP.36 Adverse reactions such as ocular hypotony, choroidal effusions, anterior uveitis, increased iris pigmentation, recurrent CME, and facial rash were seen in patients within 1 to 4 weeks after the commencement of topical latanoprost for the treatment of primary open-angle glaucoma.37 Yalvac et al. reported 2 cases of acute angle-closure glaucoma with corneal edema and shallow anterior chambers upon slit-lamp biomicroscopy in patients using latanoprost with pre-existing histories of angle-closure glaucoma.38 These symptoms were only present in the eye without a peripheral iridotomy and resolved after withdrawal of the drug. Yalvac concluded that latanoprost may result in improved flow through the ciliary muscle, causing swelling, which displaces the iris-lens diaphragm anteriorly, triggering the acute glaucoma attacks. Patients with narrow-angle glaucoma without peripheral iridotomies or individuals at high risk for CME should be monitored closely when using latanoprost and the other prostaglandin analogues.
RPE TOXICITY: CHLOROQUINE/HYDROXYCHLOROQUINE, DEFEROXAMINE
Chloroquine/Hydroxychloroquine
Chloroquine and its modified counterpart, hydroxychloroquine, were first used for the treatment and prevention of malaria. Currently, they are widely utilized around the world to treat certain autoimmune disorders, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) due to their immunosuppressive effects. Chloroquine and hydroxychloroquine produce a myriad of adverse effects, including tinnitus, hematologic disorders, liver dysfunction, and retinal toxicity. Retinal toxicity due to chloroquine was first described by Hobbs et al. in 1959 and since then, progressive retinal and RPE degeneration have been routinely demonstrated in patients taking chronic high doses of chloroquine or hydroxychloroquine.39 Chloroquine toxicity generally occurs with a daily dose greater than 250 mg with a total accumulation of 100 to 300 mg, and hydroxychloroquine toxicity occurs in patients taking more than 6.5 mg/kg/day.40,41
Chloroquine and hydroxychloroquine both have a high affinity for melanin and concentrate in melanin-containing tissues such as the iris, ciliary body, and retina/choroid.42 Symptoms of ocular toxicity include blurred vision, paracentral or central visual field scotomas, photopsias, and/or photophobia, which may precede any clinical findings.43 The typical fundus presentation of chloroquine/hydroxychloroquine toxicity is a bullseye maculopathy that can be best appreciated with FA (Figure 5). Granular pigmentary changes begin in the macula and progress to hypopigmentation in the parafoveal region. Arteriolar narrowing and disc pallor occur in late, severe disease. Cataracts and difficulties with accommodation have also been associated with chloroquine therapy but not with hydroxychloroquine.44
With early toxicity, patients may be asymptomatic, and findings on FA may be minimal. Both the ERG and EOG are usually diminished in the early stages, although there have been normal results despite visual field deficits and pigmentary changes in some patients.45 FA has little utility as a screening tool and Neubauer et al. showed little diagnostic value of EOG.46,47 Recent evidence is mounting that suggests multifocal ERG may be a valuable diagnostic tool to detect early toxicity.48
Hydroxychloroquine has largely replaced chloroquine for the treatment of autoimmune disorders in the United States, owing in part to lower toxicity and improved efficacy.49 One prospective study of 526 patients with SLE and RA who were receiving recommended dosages of the hydroxychloroquine (<6.5 mg/kg/day) demonstrated that the overall incidences of irreversible hydroxychloroquine retinopathy in SLE and RA were 0.38% and 0.5%, respectively, in patients receiving chronic treatment over 6 years.50 Unfortunately, toxicity can be irreversible and even progressive, as some patients may experience continued functional loss and depigmentation even after drug cessation.51 In addition, clearance of these drugs from the blood can take months to years after they are stopped because of their long half-lives.52,53

FIGURE 5 APPEARS LAI WW, LAM DS. CHLOROQUINE-INDUCED BULL'S EYE MACULOPATHY. HONG KONG MED J. 2005 FEB; 11(1):55-7.
Figure 5. Color photographs of the right and left fundus (top) and FA of the right and left eye (bottom) demonstrating the bullseye pattern associated with chloroquine maculopathy.
DEFEROXAMINE
Deferoxamine is a chelating agent that binds to free iron in the blood and enhances its elimination in the urine. It removes excess iron from the body in individuals requiring repeated blood transfusions secondary to systemic illnesses such as hemochromatosis and hemosiderosis. Deferoxamine toxicity may result in the rapid development of visual loss, nyctalopia, dyschromatopsia, and central and peripheral field loss along with hearing loss following intravenous (IV) and subcutaneous administration.54,55
Clinically, the fundus can appear normal or with a slight graying of the macula during the early stages; changes in the RPE may be detected with FA or FAF. Over time, RPE pigmentary changes can develop. FA may also be notable for leakage from the retinal vessels and/or optic disc in advanced stages (Figure 6).56,57 Patients with deferoxamine toxicity may also exhibit reduced ERG amplitudes and reduced EOG light-peak to dark-trough (Arden) ratios.58,59 Light and electron microscopic histologic studies show changes in the RPE that include loss of microvilli from the apical surface, patchy depigmentation, vacuolation of the cytoplasm, swelling and calcification of mitochondria, and disorganization of the plasma membrane, along with abnormal thickening of Bruch's membrane.60 Approximately 70% of patients who discontinue therapy will experience restoration of visual function.
VASCULAR TOXICITY: TALC, INTERFERON
Talc
Talc retinopathy has been well documented in illicit drug users who commonly inject a solution of crushed pills such as methylphenidate or methadone that contain talc as an inert filler. Talc particles ranging from 5 to 10 μm can embolize and lodge in the small arterioles of the retinal vasculature, predominantly in the macular area.61 There they induce endothelial cell proliferation or granuloma formation that may completely occlude the lumen and cause retinal ischemia.62 The ischemia from talc retinopathy may lead to peripheral retinal or optic disk neovascularization, vitreous hemorrhage, tractional retinal detachment, RPE abnormalities, or macular fibrosis, any of which can cause permanent loss of vision.63 Fundus examination reveals small, glistening, white punctuate lesions scattered over the posterior pole, and FA may demonstrate peripheral retinal neovascularization and/or macular ischemia.64 Treatment of retinal neovascularization usually involves panretinal laser photocoagulation and occasionally necessitates pars plana vitrectomy with endolaser.
Interferon
Interferon-α is used to treat various illnesses including chronic hepatitis B and C infection, renal cell carcinoma, leukemia, lymphoma, AIDS-related Kaposi's sarcoma, malignant melanoma, and hemangiomatosis. Interferon retinopathy is typically characterized by cotton wool spots and retinal hemorrhages in a peripapillary distribution.65,66 These findings can occur anywhere from 2 weeks to 3 months after start of therapy, may or may not be dose-dependent, and usually resolve spontaneously or disappear when the drug is discontinued.67 Most patients with interferon retinopathy are asymptomatic; however, vision loss may occur and can be irreversible in some patients even after discontinuation of therapy.68 Branch retinal artery and vein occlusion, central retinal vein with or without branch retinal artery occlusion, CME, and optic disc edema have all been associated with interferon therapy and can cause irreversible vision loss.69-71

FIGURE 6 APPEARS COURTESY OF DRS. SHANTAN REDDY AND LAWRENCE YANNUZZI.
Figure 6. Color fundus photographs (A,B) of the right and left fundi of a patient taking deferoxamine demonstrate macular pigmentary mottling. Corresponding fundus autofluorescence images (C,D) reflect hyperautofluorescent areas, suggesting impaired processing of lipofuscin by retinal pigment epithelial cells and hypoautofluorescent areas consistent with RPE atrophy. Horizontal optical coherence tomograms (C,D) reflect disruption and atrophy of the RPE cell layer.
Patients with diabetes mellitus or systemic hypertension or who are taking pegylated interferon or high interferon doses are more likely to experience interferon retinopathy and should be closely monitored when taking this drug.72 In patients with diabetes mellitus or hypertension, interferon therapy may be continued even in the event of retinopathy if the systemic benefits outweigh the ocular risks. Controlling associated hypertension alone may be enough to ameliorate the retinopathy without the need to discontinue interferon treatment.73
CHOROIDAL TOXICITY: METRONIDAZOLE, TOPIRAMATE, ESCITALOPRAM
Metronidazole
Metronidazole is widely used to treat anaerobic and protozoal infections and common dermatologic conditions such as rosacea. Metronidazole and other sulfanilamide analogues such as sulfa antibiotics, hydrochlorothiazide, and acetazolamide can cause acute bilateral transient myopia and reduction of anterior-chamber depth.74 It has been hypothesized that these changes are due to an idiosyncratic drug reaction that causes swelling of the ciliary body, resulting in a forward rotation of the lens-iris diaphragm. Perimacular folds in the absence of angiographic macular edema have also been documented. Complete reversal of ocular changes can be expected with cessation of the drug.
Topiramate
Topiramate (Topomax, Ortho-McNeil Neurologics) is an anticonvulsant agent used to treat epilepsy and prevent migraine headaches. It has also been used in an off-label fashion in the treatment of obesity, bulimia nervosa, bipolar disorder, post-traumatic stress disorder, smoking, and alcoholism. Common adverse events with this drug include psychomotor retardation, weight loss, dizziness, and cognitive dysfunction, and more serious reported side effects are osteoporosis, rickets, and visual disturbances.
In 2001, Banta and colleagues reported a case of uveal effusion and acute bilateral secondary angle-closure glaucoma associated with topiramate use that has been well documented in many cases thereafter.76,77 It has been suggested that ciliochoroidal swelling secondary to an idiosyncratic medication effect may cause forward displacement of the lens-iris diaphragm and anterior chamber shallowing, resulting in an acute myopic shift and angle-closure glaucoma.78 These symptoms most commonly present within the first 2 weeks of treatment or several hours after a rapid increase in medication dosage. Patients usually complain of blurred vision and occasionally report periocular pain or edema.79 Clinical examination shows findings consistent with glaucoma, ie, high IOP, shallow anterior chambers, corneal edema, conjunctival hyperemia, and cataract progression. Ultrasonography has been widely used to aid in the diagnosis of acute myopia, angle-closure glaucoma, and suprachoroidal effusions in these cases, but OCT can also be used to confirm narrow iridocorneal angles and monitor progress.80 Unlike most causes of angle closure, laser peripheral iridotomy typically fails to lower IOP in these cases, as the ciliochoroidal swelling and trabecular meshwork occlusion are not relieved. The appropriate management includes discontinuation of the offending drug, cycloplegia to encourage posterior rotation of the lens-iris diaphragm, and occasionally topical and/or systemic steroids in an attempt to attenuate the allergic-type drug reaction.81
In 2006, Fine and colleagues reported that escitalopram (Lexapro, Forest Pharmaceuticals) may induce acute bilateral angle closure by a similar mechanism to topiramate:82 drug-induced ciliochoroidal edema with anterior rotation of the ciliary body and secondary angle closure. Discontinuation of the drug, cycloplegia, and topical and systemic steroids resulted in resolution of the attack. Escitalopram is a selective serotonin reuptake inhibitor used in the treatment of depression; choroidal effusion leading to angle closure is a rare side effect of this commonly prescribed medication.
UVEITIS: RIFABUTIN, CIDOFOVIR
Rifabutin
Rifabutin is an antimycobacterial agent used primarily for the treatment of Mycobacterium avium-complex (MAC) in AIDS patients. It has been well documented that a small percentage of patients taking rifabutin prophylactically or for the treatment of systemic MAC in doses as low as 300 mg/day can experience uveitis, usually occurring bilaterally and anteriorly, with or without hypopyon (Figure 7), although posterior uveitis and retinal vasculitis have been encountered as well.83,84 There have also been a small number of cases of hypopyon uveitis reported in human immunovirus (HIV)-negative immunocompetent and immunosuppressed individuals with MAC pulmonary infections after the initiation of rifabutin therapy.85,86 Vaudaux et al. also described rifabutin-induced CME in addition to unilateral anterior uveitis with hypopyon in a HIV-negative patient with pulmonary MAC infection that resolved after cessation of the rifabutin.87

FIGURE 7 REPRINTED FROM OPHTHALMOLOGY, VOL. 108, BHAGAT N, READ RW, RAO NA, SMITH RE, CHONG LP. RIFABUTIN-ASSOCIATED HYPOPYON UVEITIS IN HUMAN IMMUNODEFICIENCY VIRUS-NEGATIVE IMMUNOCOMPETENT INDIVIDUALS, 750-752, 2001, WITH PERMISSION FROM ELSEVIER.
Figure 7. Slit-lamp photograph of the right eye revealing a fibrinoid reaction with a 1-mm hypopyon in the anterior chamber of a patient taking 9 months of rifabutin, clarithromycin, and ethambutol for pulmonary Mycobacterium avium complex infection.
The classic therapeutic regimen for MAC consists of rifabutin, clarithromycin, and ethambutol. However, clarithromycin and/or fluconazole can raise rifabutin levels (and consequently the risk for uveitis) due to shared hepatic metabolism by the same cytochrome P450 system.88,89 Rifabutin-induced uveitis may mimic infectious endophthalmitis, especially when a hypopyon is present; therefore, prompt evaluation is necessary. A history of high rifabutin dosage, especially in combination with clarithromycin and/or flucanozole, may be suggestive of toxicity. Discontinuation of rifabutin and initiation of steroid therapy may result in dramatic improvements in acuity and resolution of uveitis over days to weeks.
Cidofovir
Intravenous or intravitreous cidofovir is primarily used for the treatment of cytomegalovirus (CMV) retinitis usually found in AIDS patients, but it is also an effective antiherpetic agent, particularly against acyclovir-resistant strains. CMV retinitis is a common opportunistic infection associated with AIDS and occurs in 20% to 40% of individuals with AIDS and requires lifelong maintenance therapy.90 Previous therapeutic agents for CMV were primarily ganciclovir and foscarnet, but cidofovir has been shown to have similar efficacy as these agents in slowing retinitis progression with longer half-lives requiring less frequent administration and eliminating the need for an indwelling intravenous catheter.91,92 Intravitreous and IV cidofovir use has been associated with nephropathy and ocular toxicities characterized by nongranulomatous anterior uveitis, iritis, and/or hypotony.93-95 One reported case of anterior uveitis and hypotony with systemic cidofovir in an AIDS patient without CMV retinitis confirms that these adverse effects are due to the medication rather than being a result of a reaction with the CMV organism or other infectious agents since uveitis is encountered in patients with HIV due to other bacterial, fungal, viral, or protozoal pathogens.96-99
Studies have shown that intravenous cidofovir causes anterior uveitis in 26% to 44% of patients with previously treated retinitis.100 The incidence of uveitis is reportedly more prevalent in patients who received previous treatment for CMV retinitis, took protease inhibitors, had improved immune function (rising CD4+ T-cell counts), or had longstanding or relapsed retinitis.101 Patients can be asymptomatic at the time of diagnosis with no abnormal findings on slit-lamp examination, but because the exact incidence of anterior uveitis is still unknown, all patients receiving intravitreous or intravenous cidofovir should be followed with slit-lamp examinations. Studies have demonstrated that intravitreous cidofovir followed by oral probenecid results in fewer cases of uveitis with no effect on hypotony.102,103 If anterior uveitis develops with cidofovir use, prompt treatment with topical steroid and cycloplegic agents with or without stopping the offending agent can effectively resolve symptoms.104 Ocular hypotony has been seen with intravitreal doses as low as 20 μg with increased frequency in higher doses, and it is the most serious ocular side effect associated with cidofovir use, necessitating discontinuation of the medication when encountered.105,106
MISCELLANEOUS: DIGOXIN, METHANOL, SILDENAFIL, VITAMIN A DEFICIENCY
Digoxin
Digoxin (also called digitalis) was first extracted from the foxglove plant, Digitalis lanata, and is used primarily for the treatment of various cardiac conditions, including atrial fibrillation, atrial flutter, and congestive heart failure. The drug is notorious for having a small therapeutic window and can be difficult to maintain at effective but nontoxic levels. Visual abnormalities are the first and most common signs of digoxin toxicity, occurring in 7% to 20% of adults on the medication.107 Patients most frequently complain of decreased acuity, xanthopsia, chromatopsia, photopsias, photophobia, and cecocentral scotoma.108,109 The retina is believed to be the main site of digoxin toxicity. Digoxin inhibits sodium-potassium ATPases found in Muller cells, photoreceptors and the RPE, which influences the uptake of extracellular potassium and alters retinal electrical properties.110 Cone ERG studies reveal prolonged b-wave implicit times that normalize when the drug is stopped, suggesting cone dysfunction.111 All visual disturbances disappear days to weeks after digoxin is discontinued.
Some historians believe the painter Vincent Van Gogh was prescribed toxic levels of digitalis as a treatment for epilepsy. Xanthopsia could account, at least in part, for Van Gogh's extensive use of swirling yellow color in his works, epitomized by one of his most famous paintings, "Starry Night."112
Methanol
Methanol is commonly found in antifreeze, solvents, and certain fuels and is occasionally consumed by alcoholics. Methanol toxicity usually occurs after ingestion and rarely with inhalation or dermal absorption.113 After a 12- to 24-hour asymptomatic latent period, patients complain of symptoms including photophobia, blurred vision, visual field deficits, and vision loss.114,115 Methanol is broken down by the enzyme alcohol dehydrogenase in the liver, producing the metabolite formic acid, which is directly toxic to the inner retina and optic nerve and can result in permanent blindness.116 Clinical examination reveals edema of the optic disc and retina, which can later progress to optic atrophy.117 Histological examination of pathological specimens confirms methanol's direct toxic effects on the optic nerve and retina.118 Focal RPE detachments, areas of cone degeneration, and dilated subretinal spaces can be detected with light microscopy and electron microscopy reveals absent apical microvilli, degeneration or vacuolation of RPE cell apices, and enlarged retinal subspaces.119 Prompt treatment of methanol toxicity with fomepizole, ethanol, and/or hemodialysis may restore visual function.120,121
Sildenafil
Sildenafil (Viagra, Pfizer) was initially evaluated for patients with hypertension and angina but was found to have an unexpected side effect that led to its introduction as the first oral agent for erectile dysfunction. The most common side effects reported with sildenafil use are headache, flushing, dyspepsia, nasal congestion, and visual disturbances that include bluish-tinged or hazed vision, blurred vision, halos, and photophobia. These visual symptoms are not associated with loss of acuity or visual field and appear to be dose-related, occurring rarely at the lowest clinical doses (25 to 50 mg) but appearing in almost 50% of patients taking 200 mg.122 Symptoms are most prominent an hour after ingestion and disappear over 3 to 4 hours.
These visual problems can be explained by sildenafil's direct effect on the retina. Sildenafil acts as a selective inhibitor of phosphodiesterase type 5 (PDE5) in the corpus cavernosum but also has 10% effectiveness against PDE6, which is found in the retinal photoreceptors. Sildenafil causes alterations in the concentrations of cyclic guanosine monophosphate (cGMP) and influences the phototransduction cascade.123 Although high cGMP levels have been shown to be retinotoxic in humans, histologic studies on animals have demonstrated that sildenafil does not result in any outer-segment abnormalities nor any cell loss in the outer nuclear layer.124 The exact mechanism of these visual disturbances remains unknown. ERG studies to date have shown varying responses to the medication. One pilot study showed no statistically significant effects of sildenafil on cone- or rod-mediated ERG responses.125 However, other studies demonstrated increased rod sensitivity and responsiveness to light stimuli or decreased a-wave and b-wave amplitudes that peak 1 hour after ingestion of 100 mg of sildenafil with complete recovery after 6 hours.126,127
Because of the rarity and transient nature of these symptoms and no evidence of permanent retinal changes stemming from drug toxicity, there is no reason for screening retinal examinations for patients taking sildenafil. Patients should take the lowest effective dose and be cautious with driving and other visual tasks.
Vitamin A Deficiency
Vitamin A deficiency (VAD) continues to be a major world public health issue in developing and developed countries due to malnutrition and malabsorption, respectively, and it is the current leading cause of childhood blindness worldwide.128 Vitamin A plays an essential role in vision. It is necessary for conjunctival epithelial cell RNA and glycoprotein synthesis, which helps to maintain the conjunctival mucosa and corneal stroma and also acts as a precursor to photosensitive visual pigments within photoreceptors and participates in photoreceptor outer segment turnover.129 VAD, therefore, produces a wide array of ocular disorders collectively known as xerophthalmia. Bitot's spots on the conjunctiva are a pathognomonic finding in the disease. Night blindness is one of the earliest and most common findings. Retinal changes are seen in later stages. In 1928, Uyemura was the first to describe the yellow and white retinal spots characteristic of VAD in 2 young boys.130 Since then, other authors have described similar yellow/white lesions in the fundus in association with decreased vision, restricted visual fields, and/or electrophysiological abnormalities in patients with VAD (Figure 8).131-134 Replenishing vitamin A stores usually results in the recovery of vision loss and retinal spots.135,136

FIGURE 8 REPRINTED FROM RETINA, VOL. 25, APUSHKIN MA, FISHMAN GA. IMPROVEMENT IN VISUAL FUNCTION AND FUNDUS FINDINGS FOR A PATIENT WITH VITAMIN A-DEFICIENT RETINOPATHY, 650-652, 2005, WITH PERMISSION FROM LIPPINCOTT WILLIAMS & WILKINS.
Figure 8. Photograph of the left fundus (A) demonstrating multiple yellow-white punctuate lesions most apparent in the midperiphery and posterior pole with normal macula, optic disk, and retinal vessel architecture. Photograph of the same left fundus (B) showing decreased number of yellow-white lesions after 2 months following treatment with vitamin A.
CONCLUSION
Although drug-induced oculopathy is relatively rare, we have presented a number of systemic and local medications that can produce retinal or uveal toxicity when taken at standard therapeutic or toxic levels. Although the exact mechanisms of pathogenesis for many of these drugs remain unknown, there are specific retinal and uveal findings attributed to certain drugs. It is imperative that these patterns are recognized because early detection and prompt treatment are often crucial in reversing these adverse ocular effects. The associated clinical presentations and FA, OCT, ultrasonography, perimetry, ERG, and FAF are important aids for diagnosis. Retinal physicians should always include medication toxicity in the differential diagnosis when working with patients presenting with aberrant clinical ophthalmologic findings. RP
REFERENCES
- Jordan VC. Fourteenth Gaddum Memorial Lecture. A current view of tamoxifen for the treatment and prevention of breast cancer. Br J Pharmacol. 1993;110:507-517.
- Kaiser-Kupfer MI, Lippman ME. Tamoxifen retinopathy. Cancer Treat Rep. 1978;62:315-320.
- Cronin BG, Lekich CK, Bourke RD. Tamoxifen therapy conveys increased risk of developing a macular hole. Int Ophthalmol. 2005;26:101-105.
- Gualino V, Cohen SY, Delyfer MN, et al. Optical coherence tomography findings in tamoxifen retinopathy. Am J Ophthalmol. 2005;140:757-758.
- Martine MF, Joel G, Maddalena QE. Optical coherence tomography in tamoxifen retinopathy. Breast Cancer Res Treat. 2006;99:117-118.
- McKeown CA, Swartz M, Blom J, Maggiano JM. Tamoxifen retinopathy. Br J Ophthalmol. 1981;65:177-179.
- Kaiser-Kupfer MI, Kupfer C, Rodrigues MM. Tamoxifen retinopathy. A clinicopathologic report. Ophthalmology. 1981;88:89-93.
- Vinding T, Nielsen NV. Retinopathy caused by treatment with tamoxifen in low dosage. Acta Ophthalmol (Copenh). 1983;61:45-50.
- Pavlidis NA, Petris C, Briassoulis E, et al. Clear evidence that longterm, lowdose tamoxifen treatment can induce ocular toxicity. A prospective study of 63 patients. Cancer. 1992;69:2961-2964.
- Nayfield SG, Gorin MB. Tamoxifen-associated eye disease. A review. J Clin Oncol. 1996 Mar;14:1018-1026.
- Heier JS, Dragoo RA, Enzenauer RW, Waterhouse WJ. Screening for ocular toxicity in asymptomatic patients treated with tamoxifen. Am J Ophthalmol. 1994;117:772-775.
- Kopcke W, Barker FM, Schalch W. Canthaxanthin deposition in the retina: a biostatistical evaluation of 411 patient. J Toxicol Cut Ocular Toxicol. 1995;14:89-104.
- Daicker B, Schiedt K, Adnet JJ, Bermond P. Canthaxanthin retinopathy. An investigation by light and electron microscopy and physicochemical analysis. Graefes Arch Clin Exp Ophthalmol. 1987;225:189-197.
- Goralczyk R, Buser S, Bausch J, Bee W, Zuhlke U, Barker FM. Occurrence of birefringent retinal inclusions in cynomolgus monkeys after high doses of canthaxanthin. Invest Ophthalmol Vis Sci. 1997;38:741-752.
- Weber U, Goerz G, Hennekes R. Carotenoid retinopathy. I. Morphologic and functional findings. Klin Monatsbl Augenheilkd. 1985;186:351-354.
- Harnois C, Cortin P, Samson J, Boudreault G, Malenfant M, Rousseau A. Static perimetry in canthaxanthin maculopathy. Arch Ophthalmol. 1988;106:58-60.
- Boudreault G, Cortin P, Corriveau LA, Rousseau AP, Tardif Y, Malenfant M. Canthaxanthine retinopathy: 1. Clinical study in 51 consumers. Can J Ophthalmol. 1983;18:325-328.
- Weber U, Goerz G. Carotinoid retinopathy. III. Reversibility. Klin Monatsbl Augenheilkd. 1986;188:20-22.
- Scallon LJ, Burke JM, Mieler WF, Kies JC, Aaberg TM. Canthaxanthine-induced retinal pigment epithelial changes in the cat. Curr Eye Res. 1988;7:687-693.
- Arden GB, Oluwole JO, Polkinghorne P, et al. Monitoring of patients taking canthaxanthin and carotene: an electroretinographic and ophthalmological survey. Hum Toxicol. 1989;8:439-450.
- Harnois C, Samson J, Malenfant M, Rousseau A. Canthaxanthin retinopathy. Anatomic and functional reversibility. Arch Ophthalmol. 1989;107:538-540.
- Krill AE, Klein BA. Flecked retina syndrome. Arch Ophthalmol. 1965;1: 496.
- Bullock JD, Albert DM, Skinner HC, Miller WH, Galla JH. Calcium oxalate retinopathy associated with generalized oxalosis: x-ray diffraction and electron microscopic studies of crystal deposits. Invest Ophthalmol. 1974;13:256-265.
- Novak MA, Roth AS, Levine MR. Calcium oxalate retinopathy associated with methoxyflurane abuse. Retina. 1988;8:230-236.
- Bullock JD, Albert DM. Flecked retina. Appearance secondary to oxalate crystals from methoxyflurane anesthesia. Arch Ophthalmol. 1975;93:26-31.
- Centers for Disease Control and Prevention. Use of niacin in attempts to defeat urine drug testing-five states, January-September 2006. MMWR Morb Mortal Wkly Rep. 2007;56:365-366.
- Gass JD. Nicotinic acid maculopathy. Am J Ophthalmol. 1973;76:500-510.
- Fraunfelder FW, Fraunfelder FT, Illingworth DR. Adverse ocular effects associated with niacin therapy. Br J Ophthalmol. 1995;79:54-56.
- Millay RH, Klein ML, Illingworth DR. Niacin maculopathy. Ophthalmology. 1988;95:930-936.
- Dajani HM, Lauer AK. Optical coherence tomography findings in niacin maculopathy. Can J Ophthalmol. 2006;41:197-200.
- Spirn MJ, Warren FA, Guyer DR, et al. Optical coherence tomography findings in nicotinic acid maculopathy. Am J Ophthalmol. 2003;135:913-914.
- Jampol LM. Niacin maculopathy. Ophthalmology. 1988;95:1704-1705.
- Lachkar Y, Bouassida W. Drug-induced acute angle closure glaucoma. Curr Opin Ophthalmol. 2007;18:129-133.
- Classe JG. Epinephrine maculopathy. J Am Optom Assoc. 1980;51:1091- 1093.
- Michels RG, Maumenee AE. Cystoid macular edema associated with topically applied epinephrine in aphakic eyes. Am J Ophthalmol. 1975;80:379-388.
- Sagara T, Gaton DD, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN. Topical prostaglandin F2alpha treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch Ophthalmol. 1999;117:794-801.
- Rowe JA, Hattenhauer MG, Herman DC. Adverse side effects associated with latanoprost. Am J Ophthalmol. 1997;124:683-685.
- Yalvac IS, Tamcelik N, Duman S. Acute angle-closure glaucoma associated with latanoprost. Jpn J Ophthalmol. 2003;47:530-531.
- Hobbs HE, Sorsby A, Freedman A. Retinopathy following chloroquine therapy. Lancet. 1959;2:478-480.
- Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF; American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:1377-1382.
- Bernstein HN. Ocular safety of hydroxychloroquine. Ann Ophthalmol. 1991;23:292-296.
- Tanaka M, Takashina H, Tsutsumi S. Comparative assessment of ocular tissue distribution of drug-related radioactivity after chronic oral administration of 14C-levofloxacin and 14C-chloroquine in pigmented rats. J Pharm Pharmacol. 2004;56:977-983.
- Hart WM Jr, Burde RM, Johnston GP, Drews RC. Static perimetry in chloroquine retinopathy. Perifoveal patterns of visual field depression. Arch Ophthalmol. 1984;102:377-380.
- Bernstein HN. Ophthalmologic considerations and testing in patients receiving long-term antimalarial therapy. Am J Med. 1983;75:25-34.
- Tzekov R. Ocular toxicity due to chloroquine and hydroxychloroquine: electrophysiological and visual function correlates. Doc Ophthalmol. 2005;110: 111-120.
- Easterbrook M. Ocular effects and safety of antimalarial agents. Am J Med. 1988;85:23-29.
- Neubauer AS, Samari-Kermani K, Schaller U, Welge-Lubetaen U, Rudolph G, Berninger T. Detecting chloroquine retinopathy: electro-oculogram versus colour vision. Br J Ophthalmol. 2003;87:902-908.
- Moschos MN, Moschos MM, Apostolopoulos M, Mallias JA, Bouros C, Theodossiadis GP. Assessing hydroxychloroquine toxicity by the multifocal ERG. Doc Ophthalmol. 2004;108:47-53.
- Rynes RI. Antimalarial drugs in the treatment of rheumatological diseases. Br Rheumatol. 1997;36:799-805.
- Mavrikakis I, Sfikakis PP, Mavrikakis E, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology. 2003;110:1321-1326.
- Easterbrook M. Long-term course of antimalarial maculopathy after cessation of treatment. Can J Ophthalmol. 1992;27:237-239.
- Ette EI, Brown-Awala EA, Essien EE. Chloroquine elimination in humans: effect of low-dose cimetidine. J Clin Pharmacol. 1987;27:813-816.
- Estes ML, Ewing-Wilson D, Chou SM, et al. Chloroquine neuromyotoxicity. Clinical and pathologic perspective. Am J Med. 1987;82:447-455.
- Davies SC, Marcus RE, Hungerford JL, et al. Ocular toxicity of highdose intravenous desferrioxamine. Lancet. 1983;2:181-184.
- Mehta AM, Engstrom RE Jr, Kreiger AE. Deferoxamine-associated retinopathy after subcutaneous injection. Am J Ophthalmol. 1994;118:260-262.
- Lakhanpal V, Schocket SS, Jiji R. Deferoxamine (Desferal)-induced toxic retinal pigmentary degeneration and presumed optic neuropathy. Ophthalmology. 1984;91:443-451.
- Gass JMD. Toxic diseases affecting the pigment epithelium and retina. In: Gass JDM, ed. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. 4th ed. St. Louis, MO: Mosby; 1997.
- Haimovici R, D'Amico DJ, Gragoudas ES, Sokol S. Deferoxamine Retinopathy Study Group. The expanded clinical spectrum of deferoxamine retinopathy. Ophthalmology. 2002;109:164-171.
- Arden GB. Desferrioxamine administered intravenously by infusion causes a reduction in the electroretinogram in rabbits anaesthetized with urethane. Hum Toxicol. 1986;5:229-236.
- Rahi AH, Hungerford JL, Ahmed AI. Ocular toxicity of desferrioxamine: light microscopic histochemical and ultrastructural findings. Br J Ophthalmol. 1986;70:373-381.
- Jampol LM, Setogawa T, Rednam KR, Tso MO. Talc retinopathy in primates. A model of ischemic retinopathy: I. Clinical studies. Arch Ophthalmol. 1981;99:1273-1280.
- Tse DT, Ober RR. Talc retinopathy. Am J Ophthalmol. 1980;90:624-640.
- Sharma MC, Ho AC. Macular fibrosis associated with talc retinopathy. Am J Ophthalmol. 1999;128:517-519.
- Friberg TR, Gragoudas ES, Regan CD. Talc emboli and macular ischemia in intravenous drug abuse. Arch Ophthalmol. 1979;97:1089-1091.
- Guyer DR, Tiedeman J, Yannuzzi LA, et al. Interferon-associated retinopathy. Arch Ophthalmol. 1993;111:350-356.
- Cuthbertson FM, Davies M, McKibbin M. Is screening for interferon retinopathy in hepatitis C justified? Br J Ophthalmol. 2004;88:1518-1520.
- Hayasaka S, Nagaki Y, Matsumoto M, Sato S. Interferon associated retinopathy. Br J Ophthalmol. 1998;82:323-325.
- Hejny C, Sternberg P, Lawson DH, Greiner K, Aaberg TM Jr. Retinopathy associated with high-dose interferon alfa-2b therapy. Am J Ophthalmol. 2001;131:782-787.
- Rubio JE Jr, Charles S. Interferon-associated combined branch retinal artery and central retinal vein obstruction. Retina. 2003;23:546-548.
- Tokai R, Ikeda T, Miyaura T, Sato K. Interferon-associated retinopathy and cystoid macular edema. Arch Ophthalmol. 2001;119:1077-1079.
- Norcia F, Di Maria A, Prandini F, Redaelli C. Natural interferon therapy: optic nerve ischemic damage? Ophthalmologica. 1999;213:339-340.
- d'Alteroche L, Majzoub S, Lecuyer AI, Delplace MP, Bacq Y. Ophthalmologic side effects during alpha-interferon therapy for viral hepatitis. J Hepatol. 2006;44:56-61.
- Han C, O'day J. Symptomatic interferon retinopathy successfully treated by hypertension management. Br J Ophthalmol. 2007;91:1250-1251.
- Grinbaum A, Ashkenazi I, Avni I. Drug induced myopia associated with treatment for gynecological problems. Eur J Ophthalmol. 1995;5:136-138.
- Ryan EH Jr, Jampol LM. Drug-induced acute transient myopia with retinal folds. Retina. 1986;6:220-223.
- Banta JT, Hoffman K, Budenz DL, Ceballos E, Greenfield DS. Presumed topiramate- induced bilateral acute angle-closure glaucoma. Am J Ophthalmol. 2001;132:112-114.
- Sankar PS, Pasquale LR, Grosskreutz CL. Uveal effusion and secondary angleclosure glaucoma associated with topiramate use. Arch Ophthalmol. 2001;119:1210-1211.
- Rhee DJ, Goldberg MJ, Parrish RK. Bilateral angle-closure glaucoma and ciliary body swelling from topiramate. Arch Ophthalmol. 2001;119:1721-1723.
- Fraunfelder FW, Fraunfelder FT, Keates EU. Topiramate-associated acute, bilateral, secondary angle-closure glaucoma. Ophthalmology. 2004;111:109-111.
- Palomares P, Amselem L, Diaz-Llopis M. Optical coherence tomography for diagnosis and monitoring of angle-closure glaucoma induced by topiramate. Can J Ophthalmol. 2007;42:633-634.
- Chen TC, Chao CW, Sorkin JA. Topiramate induced myopic shift and angle closure glaucoma. Br J Ophthalmol. 2003;87:648-649.
- Zelefsky JR, Fine HF, Rubinstein VJ, Hsu IS, Finger PT. Escitalopram-induced uveal effusions and bilateral angle closure glaucoma. Am J Ophthalmol. 2006;141:1144-1147.
- Chaknis MJ, Brooks SE, Mitchell KT, Marcus DM. Inflammatory opacities of the vitreous in rifabutin-associated uveitis. Am J Ophthalmol. 1996;122:580-582.
- Arevalo JF, Russack V, Freeman WR. New ophthalmic manifestations of presumed rifabutin-related uveitis. Ophthalmic Surg Lasers. 1997;28:321-324.
- Bhagat N, Read RW, Rao NA, Smith RE, Chong LP. Rifabutin-associated hypopyon uveitis in human immunodeficiency virus-negative immunocompetent individuals. Ophthalmology. 2001;108:750-752.
- Jewelewicz DA, Schiff WM, Brown S, Barile GR. Rifabutin-associated uveitis in an immunosuppressed pediatric patient without acquired immunodeficiency syndrome. Am J Ophthalmol. 1998;125:872-873.
- Vaudaux JD, Guex-Crosier Y. Rifabutin-induced cystoid macular oedema. J Antimicrob Chemother. 2002;49:421-422.
- Saran BR, Maguire AM, Nichols C, et al. Hypopyon uveitis in patients with acquired immunodeficiency syndrome treated for systemic Mycobacterium avium complex infection with rifabutin. Arch Ophthalmol. 1994;112:1159-1165.
- Tseng AL, Walmsley SL. Rifabutin-associated uveitis. Ann Pharmacother. 1995;29:1149-1155.
- Hoover DR, Peng Y, Saah A, et al. Occurrence of cytomegalovirus retinitis after human immunodeficiency virus immunosuppression. Arch Ophthalmol. 1996;114:821-827.
- Lalezari JP, Drew WL, Glutzer E, et al. (S)-1-[3-hydroxy-2-(phosphonylmethoxy) propyl]cytosine (cidofovir): results of a phase I/II study of a novel antiviral nucleotide analogue. J Infect Dis. 1995;171:788-796.
- Lalezari JP. Cidofovir: a new therapy for cytomegalovirus retinitis. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14(Suppl 1):S22-S26.
- Chavez-de la Paz E, Arevalo JF, Kirsch LS, et al. Anterior nongranulomatous uveitis after intravitreal HPMPC (cidofovir) for the treatment of cytomegalovirus retinitis. Analysis and prevention. Ophthalmology. 1997;104:539-544.
- Kirsch LS, Arevalo JF, De Clercq E, et al. Phase I/II study of intravitreal cidofovir for the treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1995;119:466-476.
- Tseng AL, Mortimer CB, Salit IE. Iritis associated with intravenous cidofovir. Ann Pharmacother. 1999;33:167-171.
- Scott RA, Pavesio C. Ocular side-effects from systemic HPMPC (Cidofovir) for a non-ocular cytomegalovirus infection. Am J Ophthalmol. 2000;130:126-127.
- Heinemann MH, Bloom AF, Horowitz J. Candida albicans endophthalmitis in a patient with AIDS. Case report. Arch Ophthalmol. 1987;105:1172-1173.
- Carney MD, Combs JL, Waschler W. Cryptococcal choroiditis. Retina. 1990;10:27-32.
- Dugel PU, Rao NA, Forster DJ, Chong LP, Frangieh GT, Sattler F. Pneumocystis carinii choroiditis after long-term aerosolized pentamidine therapy. Am J Ophthalmol. 1990;110:113-117.
- Davis JL, Taskintuna I, Freeman WR, Weinberg DV, Feuer WJ, Leonard RE. Iritis and hypotony after treatment with intravenous cidofovir for cytomegalovirus retinitis. Arch Ophthalmol. 1997;115:733-737.
- Ambati J, Wynne KB, Angerame MC, Robinson MR. Anterior uveitis associated with intravenous cidofovir use in patients with cytomegalovirus retinitis. Br J Ophthalmol. 1999;83:1153-1158.
- Chavez-de la Paz E, Arevalo JF, Kirsch LS, et al. Anterior nongranulomatous uveitis after intravitreal HPMPC (cidofovir) for the treatment of cytomegalovirus retinitis. Analysis and prevention. Ophthalmology. 1997;104:539-544. Erratum in: Ophthalmology. 1997;104:1063.
- Banker AS, Arevalo JF, Munguia D, et al. Intraocular pressure and aqueous humor dynamics in patients with AIDS treated with intravitreal cidofovir (HPMPC) for cytomegalovirus retinitis. Am J Ophthalmol. 1997;124:168-180.
- Akler ME, Johnson DW, Burman WJ, Johnson SC. Anterior uveitis and hypotony after intravenous cidofovir for the treatment of cytomegalovirus retinitis. Ophthalmology. 1998;105:651-657.
- Rahhal FM, Arevalo JF, Munguia D, et al. Intravitreal cidofovir for the maintenance treatment of cytomegalovirus retinitis. Ophthalmology. 1996;103:1078-1083.
- Kirsch LS, Arevalo JF, De Clercq E, et al. Phase I/II study of intravitreal cidofovir for the treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1995; 119:466-476.
- Shapiro S, Slone D, Lewis GP, Jick H. The epidemiology o digoxin. A study in three Boston hospitals. J Chronic Dis. 1969;22:361-371.
- Lely AH, van Enter CH. Large-scale digitoxin intoxication. Br Med J. 1970;3:737-740.
- Butler VP Jr, Odel JG, Rath E, et al. Digitalis-induced visual disturbances with therapeutic serum digitalis concentrations. Ann Intern Med. 1995;123:676- 680.
- Burke JM, McKay BS. In vitro aging of bovine and human retinal pigment epithelium: number and activity of the Na/K ATPase pump. Exp Eye Res. 1993;57:51-57.
- Weleber RG, Shults WT. Digoxin retinal toxicity. Clinical and electrophysiological evaluation of a cone dysfunction syndrome. Arch Ophthalmol. 1981;99:1568-1572.
- Wolf P. Creativity and chronic disease: Vincent van Gogh (1853-1890). Western J Med. 2001;175: 348.
- Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA; American Academy of Clinical Toxicology Ad Hoc Committee on the Treatment Guidelines for Methanol Poisoning. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol. 2002;40:415-446.
- Eells JT. Methanol. Thurman RG, Kaufman FC, eds. Browning's Toxicity and Metabolism of Industrial Solvents. Amsterdam, Netherlands: Elsevier; 1992:3-15.
- Hayreh MS, Hayreh SS, Baumbach GL, et al. Ocular toxicity of methanol: an experimental study. Merigan W, Weiss B, eds. Neurotoxicity of the Visual System. New York, NY: Raven Press; 1980:35-53.
- Sharpe JA, Hostovsky M, Bilbao JM, Rewcastle NB. Methanol optic neuropathy: a histopathological study. Neurology. 1982;32:1093-1100.
- Treichel JL, Henry MM, Skumatz CM, Eells JT, Burke JM. Antioxidants and ocular cell type differences in cytoprotection from formic acid toxicity in vitro. Toxicol Sci. 2004;82:183-192.
- Eells JT. Methanol. Thurman RG, Kaufman FC, eds. Browning's Toxicity and Metabolism of Industrial Solvents. Amsterdam, Netherlands: Elsevier; 1992:3-15.
- Treichel JL, Murray TG, Lewandowski MF, Stueven HA, Eells JT, Burke JM. Retinal toxicity in methanol poisoning. Retina. 2004;24: 309-312.
- Brent J, McMartin K, Phillips S, Aaron C, Kulig K. Methylpyrazole for toxic alcohols study group: fomepizole for the treatment of methanol poisoning. N Engl J Med. 2001; 8;344:424-429.
- Megarbane B, Borron SW, Trout H, et al. Treatment of acute methanol poisoning with fomepizole. Intensive Care Med. 2001;27:1370-1378.
- Food and Drug Administration Joint Clinical Review. Study 148-004: Phase I Investigatorblind, Placebo-controlled, Evaluation of Safety, Toleration, and Pharmacokinetics of Sildenafil Following Escalating Single Oral Doses in Healthy Male Volunteers. Washington, DC: FDA.
- Marmor MF, Kessler R. Sildenafil (Viagra) and ophthalmology. Surv Ophthalmol. 1999;44:153- 162.
- Wallis RM, Leishman D, Pullman L, Graepel P, Heywood R, Effects of sildenafil on retinal histopathology and electroretinogram (ERG) in dogs (abstract). Ophthalmic Res. 1998;30:S68.
- Stockbridge N. Amendment to clinical review of 22 January 1998: Effects of sildenafil on the electroretinogram in Study 148-232 [memorandum]. http://www.fda.gov/cder/foi/nda/98/viagra/ergmemo.pdf. Published 1998. Accessed December 1, 2007.
- Vobig MA, Klotz T, Staak M, Bartz-Schmidt KU, Engelmann U, Walter P. Retinal side-effects of sildenafil. Lancet. 1999;353:375.
- Gabrieli CB, Regine F, Vingolo EM, Rispoli E, Fabbri A, Isidori A. Subjective visual halos after sildenafil (Viagra) administration: Electroretinographic evaluation. Ophthalmology. 2001;108:877-881.
- World Health Organization. Prevention of Childhood Blindness. Geneva, Switzerland: World Health Organization; 1994.
- Hoyt CS, 3rd. Vitamin metabolism and therapy in ophthalmology. Surv Ophthalmol. 1979;24:177-190.
- Uyemura M. Uber eine merkwurdige augenhintergrundveranerung bie zwei fallen vo idiopathischer hemerlopie. Klin Monatsbl Augenheilkd. 1928;81:849-850.
- Apushkin MA, Fishman GA. Improvement in visual function and fundus findings for a patient with vitamin A-deficient retinopathy. Retina. 2005;25:650-652.
- Fells P, Bors F. Ocular complications of selfinduced vitamin A deficiency. Trans Ophthalmol Soc U K. 1970;89:221-228.
- Fuchs A. White spots of the fundus combined with night blindness and xerosis (Uyemura's syndrome). Am J Ophthalmol. 1959;48:101-103.
- Smith J, Steinemann TL. Vitamin A deficiency and the eye. Int Ophthalmol Clin. 2000;40:83-91.
- Bors F, Fells P. Reversal of the complications of self-induced vitamin A deficiency. Br J Ophthalmol. 1971;55:210-214.
- Sommer A, Tjakrasudjatma S, Djunaedi E, Green WR. Vitamin A-responsive panocular xerophthalmia in a healthy adult. Arch Ophthalmol. 1978;96:1630-1634.








