CONTINUING MEDICAL EDUCATION
Innovative Approaches to Small-gauge Vitrectomy Surgery
Learn about recent advances, the latest techniques and unique strategies for the best surgical outcomes.
Highlights from a roundtable discussion in September 2007 at the AVTT meeting in Chicago.
Exploring Techniques to Facilitate Small-gauge Vitrectomy
David S. Boyer, MD, (moderator): Today, we will discuss the state-of-the-art in retinal vitreous surgery, including recent advances, innovative approaches and strategies for overcoming and avoiding complications.
Some physicians dismiss the idea of using 23-g and 25-g instrumentation for vitrectomy, and even refer to it as a gimmick. The members of this panel, all experts in this field, have achieved wonderful results with 20-g instrumentation. So first let me ask: What caused you to switch to 25-g or 23-g instrumentation?
INITIAL RESERVATIONS
Steve Charles, MD: Early on, I had some biases. I thought using small-gauge instruments would be more difficult for traction detachments, proliferative vitreoretinopathy (PVR) and vitreous hemorrhages. I thought it would rule out the use of silicone and raise major wound-leak issues.
All of my assumptions proved to be incorrect. Now, I believe 23-g and 25-g instrumentation offer advantages in fluidics, providing retinal stability in all of the surgeries I just mentioned. The benefits of patient comfort are important. However, anatomic and visual outcomes, which are superior after this surgery, are even more important.
Timothy G. Murray, MD, FACS: We tried 25-g and 23-g technology at the Bascom Palmer Eye Institute because we wanted to evaluate its potential impact on surgical management. Like Dr. Charles, I also had initial reservations. My interest was less in improving patient comfort and more in enhancing the surgical procedure and potentially a more controlled environment for surgery.
I was not an early adopter, and I have not been a strong proponent of 25-g surgery. However, I believe the 23-g system addresses many of my initial concerns, which have been related to instrument availability, handling, wide-field viewing and illumination issues. I was concerned about sclerotomy site closure, which I have found is not a significant issue in either 25-g or 23-g surgery. So what began as an evaluation of new technology has now become an integral part of our surgical armamentarium.

The 23-g system addresses many of my initial concerns, which have been related to instrument availability, handling, wide-field viewing and illumination. — Timothy G. Murray, MD, FACS |
Allen C. Ho, MD, FACS: The surgeon clearly benefits from these smaller-gauge systems compared to the more invasive systems. Our primary motivation at Wills Eye Institute, though, has been to determine how small-gauge procedures, or microsurgery, might benefit patients. We have been pleased with the results. The capabilities of the systems have evolved significantly, specifically the rigidity of instrumentation and the lighting.1
MATTERS OF PREFERENCE
Dr. Boyer: Do you routinely choose one system over the other?
Dr. Charles: I exclusively use 25-g, which has demonstrated good results not just in macular surgery but in all procedures I perform.2,3
Dr. Murray: I don't disagree with Dr. Charles often. However, in this case I do. I perform 23-g surgery exclusively. I have been unhappy even with the advances in 25-g instrumentation, and I am unwilling to accept some of the trade-offs associated with that technology.
Dr. Ho: I use a blend of 23-g and 25-g systems, depending on the pathology I'm addressing. The 25-g instrumentation has excessive — although improved — flexibility. The learning curve for 25-g surgery is inside the eye because of the flexibility, lower illumination and reduced flow (or more controlled cutting, depending on how you view this aspect of the procedure). The learning curve for 23-g surgery is outside of the eye, where wound construction technique is so critical. Surgery with 23-g instruments, however, is very similar to surgery with 20-g instruments.

Surgeons and patients benefit from the use of smaller-gauge systems, such as 23-g (shown here).
STABILIZING THE EYE
Dr. Boyer: One of the challenges of converting from 25-g to 23-g, or even from 20-g to 23-g, is the entry wound. At the beginning and at the end of the procedure, we face the highest risk of complications, including retinal tears, endophthalmitis and hypotony. How do you stabilize the eye while making your incisions and displacing the conjunctiva?
Dr. Charles: The first-generation 25-g trocar-cannulas had high insertion force, which created stabilization issues. The second-generation trocar-cannulas have low insertion force, roughly equivalent to that of a 20-g microvitreoretinal (MVR) blade, so stabilization of the eye is no longer a problem.
I use a cotton-tipped applicator to displace the conjunctiva and hold the eye in position. After displacing the conjunctiva and laying the blade on the eye, I drop the cotton tip and use the forefinger of my free hand to triangulate. This way, I support the ferrule of the trocar with a two-handed approach.
This keeps the eye very stable, preventing rotation or displacement, and it lets me continue with a near-tangential approach. Initially, like most surgeons using the straight-in system, I had a few wound leaks, although no endophthalmitis, and so I developed the notion of fluid-air exchange. Other surgeons have conducted short comparison trials that appear to show that fluid-air exchange made no difference in outcomes.
I was elated when the angulated scleral tunnel wound was developed. This wound architecture is necessary when using 23-g instrumentation. I have been using it for my procedures for slightly more than a year. Every wound is constructed with very tangential initial trajectory. I tilt up, using a technique that some surgeons call "supination." It really has nothing to do with supination and does not construct a biplanar incision. However, the purpose is to avoid impaling the retina with the trocar.
SELF-SEALING ISSUES
Dr. Boyer: Has anyone encountered issues with wound construction in 23-g and 25-g surgeries?
Dr. Murray: After using the first-generation 25-g instruments to perform sclerotomies, I found that the self-sealing component was missing. Fortunately, because of wound construction research by Peter K. Kaiser, MD, we can better understand how to establish these wounds more effectively.
For 23-g surgery, I use either a cotton-tipped applicator or a toothed forceps to displace the conjunctiva in a radial rotation. I enter at a typical 30° angle, engage the sclera with the blade turned upward and enter the sclera until I encounter resistance. I then rotate again to enter the eye. With this approach, I do not have a concern about engaging peripheral or posterior retina. Globe displacement is relatively minimal.
If I use a cotton-tipped applicator, I drop the applicator and then, when removing the needle component of the trocar, I stabilize the cannula with a toothed forceps. I place my infusion cannula line first to enable me to visualize and also stabilize the eye with infusion. Once I have established control of fluidics, I make my supranasal and supratemporal again with that same displacement technique.
Initially, I found that I rotated into essentially the same direct surgical plane that I had used with an MVR blade after I had engaged the sclera. Now, I do not need to rotate as much. If you place these cannulas appropriately, you benefit from an oblique orientation to the sclera when exiting the eye in the proper position. This lets you establish a very stable exit wound with stable intraocular pressure immediately after surgery and during the first postoperative day.
IMPORTANCE OF WOUND CONSTRUCTION
Dr. Ho: Stabilizing the eye is the first step in creating a reliable, self-sealing wound. In my hands, it is very different for 25-g versus 23-g systems. If you create an appropriately angled wound, the forces for 23-g entry are significantly greater than the forces for 25-g entry. These forces not only rotate the eye circumferentially but also in a posterior direction, deep into the orbit. So my fixation techniques have evolved in a couple of ways.

I was elated when the angulated scleral tunnel wound was developed. This wound architecture is necessary when using 23-g instrumentation. — Steve Charles, MD |
For 25-g, all I need are forceps to grab at the limbus or cotton-tipped applicators to stabilize the globe to create an angled incision. Also, I try to pass the bevel of the trocar as flat as I would pass a scleral suture by a first scleral buckle. I strive for very flat penetration initially so that I can seal within the scleral tissue itself.
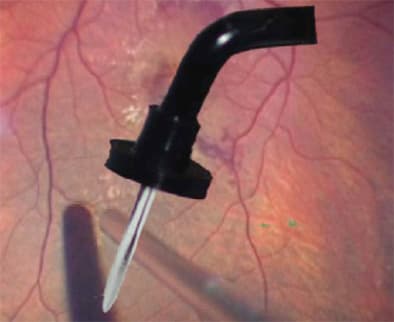
For 23-g, I almost exclusively use the Thornton Fixation Ring, a swiveled, semi-circular ring with a handle that cataract surgeons use to stabilize the eye during clear cornea procedures. The ring helps me fixate the globe when significant 23-g forces rotate it in an anterior-posterior direction. I then displace conjunctiva by grabbing the conjunctiva with the tip of the trocar, moving the conjunctiva and going flat into the eye with an angled incision through the sclera.

The Thornton Fixation Ring (shown here), a semi-circular ring helps fixate the eye during 23-g vitrectomy surgery.
AN ISSUE OF FIXATION
Dr. Murray: It is true that we have made progress with these forms of microsurgery, as everyone on this panel has made clear. However, we still have not developed a consensus on the best instrumentation for 23-g entry. Although the new generation of cannulated trocars is superior, fixation of the sclera and the globe is not yet ideal. New instrumentation that will improve how we stabilize the globe and enter the eye may be available within a relatively short time. This could help significantly.
Dr. Boyer: I agree. Recently, as surgeons have started to adopt these smaller microsurgery systems, 23-g more than 25-g, they have had to adjust to the weight of the infusion cannula, moving the tip of the infusion. If you do not fixate that correctly, you could end up with degrees of subchoroidal or subretinal infusion. If you recognized this development, how would you deal with it?

The capabilities of the [smaller gauge] systems have evolved significantly, specifically the rigidity of instrumentation and the lighting. — Allen C. Ho, MD, FACS |
Dr. Charles: We have experienced this while using 20-g infusion cannulas. If you cannot see the infusion port with the operating microscope, do not turn it on. Infuse with a longer device. With 25-g, I am comfortable simply inserting the infusion port and turning it on without looking in most eyes. The exceptions would be cases involving:
■ Hypotony
■ Suprachoroidal hemorrhages
■ Trauma
■ Pars plana scar tissue that might cause you to be in the suprachoroidal or subretinal space when you enter.
One thing that is helpful about the 25-g or any other cannula-based system is that you can change the infusion port to the other side of the eye. The choroidal infusion will normally subside without your taking further action.
Dr. Murray: I try to be preemptive. I do not think you should turn on your infusion in the eye until you visualize the cannula, which may become displaced even after visualization. We will tape the infusion tube into a position that stabilizes the cannula more rigidly, making it less likely to move.
Dr. Charles: One also should make sure the infusion cannula port is not too far below the 3 o'clock and 9 o'clock positions to keep it from hitting the lower lid and flipping into the subconjunctival space. In addition, we tape down the service loop to secure the tubing, which needs to be long enough to keep it from becoming taut and changing the position of the infusion cannula during large angles of rotation. RP
References
|
Preventing Complications Associated With Micro-incision Vitrectomy Surgery
Dr. Boyer: We have heard of the problems that can arise if small-gauge retinal surgery is not properly handled. I would like to explore these issues, beginning with air in the anterior chamber. Dr. Charles, you maintain that we can introduce air or gas into the anterior chamber by creating our sclerotomy incisions too anteriorly, making them 3 mm to 3.5 mm from the limbus. Why is that the case?
WHY AIR IS AN ISSUE
Dr. Charles: When I began using 25-g instruments, as soon as I performed fluid-air exchange, I found that a big bubble of air would move into the anterior chambers of some patients who had normal lenses and no zonular dehiscence. Because the 25-g cannulas are so much smaller than the 20-g cannulas, I concluded I was getting too comfortable moving them anteriorly. Since then, I have modified my approach, using a 4-mm wound for pseudophakic and phakic eyes and a 3-mm wound for aphakic eyes.
Dr. Boyer: Another concern is the risk of retinal tears, which many surgeons believe increases with small-gauge surgery. Dr. Ho, do you enter the eye differently with a 23-g or 25-g system than you do with a 20-g system?

Sometimes, even under the best conditions, the cannulas can become displaced, coming out attached to some of the instruments, such as the cutter or forceps. — David S. Boyer, MD |
Dr. Ho: No. When I enter with the infusion cannula, I do not turn it on until I can verify its position by looking with the light pipe externally and then examining the position internally. Theoretically, the cannula systems afford some protection against retinal tears. Many surgeons are overly concerned with the Minnesota group's report1,2 at the 2005 meeting of the American Society of Retina Specialists, the one related to increased retinal tears and endophthalmitis associated with 25-g surgery. But even the researchers now acknowledge that these increased risks may not be present any more in their setting.
To avoid these problems, we inspect the eye at the end of each case with a binocular indirect ophthalmomicroscope (BIOM) and surgeon-controlled depression to ensure the patient does not have a retinal tear. I have not seen an increase in retinal tears using either 25-g or 23-g systems.
PROTECTING AGAINST TEARS
Dr. Murray: I always have felt that the trocar systems associated with 23-g and 25-g instruments inherently protect against retinal tears. I think the greatest concern is not to incarcerate vitreous into the trocar when entering the eye and, especially, when exiting with an instrument.
My fellows and I try to keep the eye in a closed system so that we do not move vitreous through the cannulas when we exit. As an alternative, we will turn off the infusion and close our cannulas with plugs when we exit.
I've been impressed with the concept of closing the lumen as you exit the sclera. With the light pipe in place, we can extrude the cannula, ensuring there is no ostium as we exit the sclera. I have not had issues related to sclerotomy site tears. One time, the infusion cannula was distorted enough that it contacted the retina posteriorly, causing a tear. We avoid that problem now by visualizing the peripheral retina before exiting the eye.
Dr. Charles: If anything, cannulas reduce the risk as long as you are very careful. Early on, advocates of 25-g sutureless surgery said vitreous should be used to plug the wounds. I think that is incorrect. As Frank Koch, MD, has shown from endoscopy, you cannot remove all the vitreous from the wounds even though you should try.
Regarding the Minnesota paper, I would say that not all techniques are the same. Surgeons will create retinal tears if they use excessive flow rates and pull vitreous to the port instead of moving the port to the vitreous.
If you take the port to the vitreous — performing what I call "continuous engage and advance" &mdash you will be more efficient and able to remove the vitreous more safely. We can ereduce the incidence of iatrogenic retinal breaks by keeping the cut rate high and the flow rate modest and taking the port to the vitreous.
DISPLACED TROCARS
Dr. Boyer: Sometimes, even under the best conditions, the cannulas can become displaced, coming out attached to some of the instruments, such as the cutter or forceps. What are your thoughts on how to prevent this from happening, and how do you handle the problem when it occurs?
Dr. Ho: In my hands, it occurs more frequently with the polyamide cannulas of the 25-g system. I recognized that it occurred commonly when I injected agents into the eye with a 25-g needle. So first, recognize that some 25-g caliber instrumentation is slightly larger than the 25-g cannulas. By using the needle to inject intravitreal triamcinolone acetonide (Kenalog, Bristol-Myers Squibb) into the eye, for example, you can engage and pull out the cannula if you push that needle all the way to the end of the hub.
Second, even if you are using instruments that should not do this based on anatomic size — the light pipe and the cutter, for example — you still must carefully observe the cannula while removing these instruments. If you see it starting to come out, keep the instrument in the eye and push the cannula system over the instrument itself. The correct path into the eye will remain intact. If the cannula comes out, identify the displaced conjunctival wound and put the cannula over the instrument — the cutter outside of the eye, for example — then engage and enter into the displaced conjunctival wound. Try to slide it back over to the scleral wound so that you will still have displaced conjunctiva. Insert the solid instrument, and then push the cannula over to find the vitrectomy. When that is not possible — if you get subconjunctival fluid and ballooning of the conjunctiva, for example — then you must reposition the cannula.
BALLOONING OF THE CONJUNCTIVA
Dr. Boyer: If the conjunctiva balloons at that point, do you open and put in one stitch and then insert another cannula?
Dr. Ho: I will do that occasionally, but not typically. I will use a wound closure massage technique. It takes up to 30 seconds, which is an inordinately long time compared to how we used to just pull the cannulas and go, but this massaging technique can sometimes stop the leak.

I always have felt that the trocar systems associated with 23-g and 25-g instruments inherently protect against retinal tears. — Timothy G. Murray, MD, FACS |
Dr. Murray: I think this problem is more associated with 25-g than with 23-g. I have been fortunate to not have experienced full displacement of any of the 23-g cannulas.
Partial displacement can move the instrument into the suprachoroidal space. If you feel any unexpected resistance when passing the instrument through the cannula, do not advance the instrument. Evaluate your trocar positions.
Dr. Charles: I agree completely, and I would like to add two points. First, I see the cannula being dislodged most often in patients with Marfan's syndrome and in high myopes, both of whom have incredibly thin scleras. When you use scissors and forceps, carefully close them when you take them out. Second, use a cotton tip to roll out the chemosis and find the scleral wound for reinsertion.
Dr. Murray: The solid steel 23-g prototype cannulase were much more likely to displace. Placing and removing the plugs was difficult. However, as you become more comfortable using the second-generation 23-g cannulas, significant displacement is very unlikely.

Surgeons will create retinal tears if they use excessive flow rates and pull vitreous to the port instead of moving the port to the vitreous. — Steve Charles, MD |
| Suturing Sclerotomies When Using Silicone Oil |
|---|
| David S. Boyer, MD: When you use silicone oil, do you routinely use sutures? Steve Charles, MD: No, I suture as needed, even with silicone oil. But I use angulated wound construction with 25-g procedures. Timothy G. Murray, MD, FACS: I'm not comfortable placing or removing silicone oil with a cannulated micro-incision system. I believe those wounds need to be sutured. Allen C. Ho, MD, FACS: I lean toward suturing sclerotomies. I have had cases of subconjunctival silicone oil. Dr. Murray: I understand that you are placing intravitreal air in your eyes for a partial tamponade. However, I am uncomfortable placing air into eyes that do not need a tamponade. I do not think it is necessary in 23-g systems. This problem has shifted me away from 25-g. Dr. Ho: In 25-g systems, there is less of a need for placing intravitreal air into eyes for a partial tamponade. However, in 23-g systems, just that extra size will create some leaks. The behavior of the conjunctiva over the ostium should dictate whether you need to use air. I do not have any scientific data to back that up. It's just a clinical impression. Dr. Charles: Since I started using angulated wounds, I have given up using intravitreal air the way I once used it. |
FRAGMENTATION
Dr. Boyer: How do you manage a patient when you have to use the phaco fragmenter? For example, how do you handle a case with lens fragments on the retina that you believe are hard enough to warrant the use of a fragmenter?
Dr. Charles: I call that 20-25-g surgery. I do the full 25-g vitrectomy, using the standard method, then I pull the supratemporal cannula out. I make a little snip in the conjunctiva so I can see the scleral opening that was made for 25-g. I use that as a pilot hole, take the 20-g MVR blade and enlarge that wound to avoid making an additional wound.
I insert the fragmenter, raise the infusion to 65 mm Hg and toggle it up and down with the VGFI (vented/gas forced infusion), because I know I will have a huge flow rate in a 20-g fragmenter when I have an occlusion break. I use the linear setting on the Accurus (Alcon Inc., Fort Worth, Texas), which enables me to proportionally control the vacuum to pick up the lens material and take it in the mid-vitreous cavity, where I then turn on the ultrasound and proportionally increase the sonic power so I do not bounce the nuclear fragments off the tip of the fragmenter.

[When I use silicone oil], I lean toward suturing sclerotomies. I have had cases of subconjunctival silicone oil. — Allen C. Ho, MD, FACS |

About 70% of those eyes [with dislocated nuclear fragments] can be managed with the 23-g system alone, without having to open an additional sclerotomy. — Timothy G. Murray, MD, FACS |
I continue to toggle the infusion up and down, depending on whether I am actually aspirating the fragments. I do not want 65 mm Hg IOP all the time, particularly when dealing with a recent cataract wound.
MANAGING 23-G ALONE
Dr. Murray: At Bascom Palmer, we have performed surgery on 20 consecutive eyes with dislocated nuclear fragments from cataract surgery. The micro-incision system allows us to enter those eyes, insert a transconjunctival trocar and cannulate the eye. About 70% of those eyes can be managed with the 23-g system alone, without having to open an additional sclerotomy.
Dr. Boyer: If you operate on an eye that has undergone recent cataract surgery, the tangential pressure needed to put the cannula in can open up the cataract wound. Therefore, I might put a stitch in the cataract wound that I can remove at the end of the case. What do you do Dr. Murray?
Dr. Murray: I suggest routinely suturing all wounds, at least when you are starting. It enables you to stabilize IOP for that initial entry, helping you to avoid iris prolapse or incarceration. RP
References
|
The Engineering of Micro-incision Vitrectomy Surgery Systems
Dr. Boyer: Much of the success of micro-incision vitrectomy surgery systems is determined by engineering. Dr. Charles, you are an engineer. Can you discuss the flow characteristics of the 25-g and 23-g systems?
FLOW CHARACTERISTICS
Dr. Charles: The resistance to flow in a 23-g system is less than it is in a 25-g system. When comparing identical cut rates, you will find 25-g has a lower flow rate than 23-g. Alcon's 25-g cutter produces 1500 cuts per minute; the 23-g cutter, 2500 cuts per minute. At those settings, though, the flow rates are exactly the same, with identical vacuum levels.
You have to factor in cutting rate because the cutter interrupts the flow through the port and creates what I call "port-based flow limiting." If you are treating a very dense vitreous hemorrhage, you will go faster with 23-g. However, it has never been a limiting factor for me, either in diabetic traction detachment or in vitreous hemorrhage. I use 600 mm Hg vacuum and 25-g at 1500 cuts per minute.
Clearly, you have to make some technique changes, such as when injecting and removing silicone oil. I use the 1000-centistoke silicone oil. However, I do not see the fluidics of 25-g as a disadvantage. Others disagree and prefer the higher flow rates, which I strongly believe are unsafe.
I am concerned about pulsatile vitreal retinal traction and iatrogenic breaks. I like to be able to trim off the flap of a horseshoe tear and see virtually no retinal movement.
DUTY CYCLE
Dr. Boyer: Does duty cycle affect flow?
Dr. Charles: Duty cycle — the fraction of port opening vs. port closing time — does influence flow. The duty cycle changes with respect to different cutting rates in disposable pneumatic cutters, which is good because the impedance of a load should match the impedance of the source.
Impedance matching allows the most efficient transfer of power. We have a dynamic range of properties in the materials we remove from the eye. We are not in a lab removing saline. If we were doing that, instead of managing pulsatile floats through a port, we could get up at a podium and say, "The 50-50 duty cycle of the electric cutter is the best." But in the real world, it is not the best. It would be like saying only one gear in your car is ideal.
Dr. Boyer: One early myth about small-gauge surgery was that we were leaving behind large amounts of vitreous. Dr. Ho, when you perform vitrectomy, do you leave more than you did when you were using 20-g instruments?

For a macular hole (top), Allen C. Ho, MD, FACS, uses 23-g, because he depresses and shaves down vitreous to eliminate it completely. For a macular pucker (bottom), he performs a complete vitrectomy, but without shaving the base.
Dr. Ho: No. From my perspective, success is based on surgeon preference, not which system the surgeon uses. For example, in a macular hole case, I choose a 23-g system because I depress and shave down vitreous to eliminate it completely, achieve a full gas fill and reduce the chance of a subsequent tear from the gas pulling on the vitreous.
On the other hand, for macular pucker, I perform a complete vitrectomy, but without shaving the base. I am more apt to use the 25-g system, leaving slightly more vitreous. I do not have to deal with the flexibility of the instrumentation going anteriorly.
CLEAR DIFFERENCES
Dr. Murray: When considering 20-g vs. 23-g vs. 25-g, I find 25-g involves distinct learning curves, including how to effectively remove a large amount of both posterior and anterior vitreous.
To me, the 23-g system is essentially identical to the 20-g system. I handle the vitreous and remove the posterior hyaloid in essentially the same way. To avoid leaving residual vitreous in these eyes, I do a very aggressive pars plana vitrectomy. One of the early complications associated with 25-g surgery was leaving behind a significant amount of vitreous. I believe residual vitreous actually may be associated with the higher risk of iatrogenic-related retinal breaks.
Dr. Charles: Avoiding residual vitreous with 25-g clearly requires technique change. In cataract surgery, surgeons learn to pull lens material centrally from the capsular recess and work on it in the middle of the capsular bag with their phacoemulsification tips. However, with the vitreous cutter, you have to take the cutter to the vitreous instead of pulling the vitreous to the cutter.
CONSIDERING NEW PORT DESIGN
Dr. Boyer: As we know, the port on the 23-g cutter is closer to the tip. Has this new design reduced your need for scissors or changed any of your other approaches?
Dr. Murray: The new design helps me engage and cut tissue better. Even with the high-speed 20-g system, I see a clear trend away from using scissors for dissection and for our more complex cases.

Because the port on the 23-g cutter is closer to the tip, you can cut tissue better, and fluidics offer more stability/control.
| Choosing Forceps |
|---|
| David S. Boyer, MD: When you need forceps for a procedure, which do you use? Steve Charles, MD: I use DSP ILM forceps (Alcon Inc.) for everything. The so-called "end-grasping" forceps do not have the same geometry. Timothy G. Murray, MD, FACS: I have been amazed by the advances in disposable instrumentation. The maintenance of nondisposable instruments has been difficult, even at an institution like Bascom Palmer. I switched entirely to disposable instrumentation, making the ILM forceps my instrument of choice. Occasionally, when I need end-grasping forceps, I will use a nondisposable instrument. With both the 25-g and the 23-g systems, though, the accessibility of instruments and the variety of instruments are limited. I look forward to expansion of the armamentarium of instruments, both for 23-g and 25-g surgery. Allen C. Ho, MD, FACS: I agree with Drs. Murray and Charles. I almost exclusively use the 25-g DSP, even for 23-g and 20-g cases. The design, reproducibility, reliability and grasp rate on difficult tissue continue to exceed my expectations for disposable instrumentation. Occasionally, I will use larger, reusable forceps, such as when I need to grasp the capsular bag to remove it, or I have a dropped intraocular lens and I need something with a little more substance. I will use the 20-g forceps in those cases. |
Dr. Ho: I agree. With the opening of the port closer to the tip, we have found that the fluidics offer more stability and control. We can get along the surface of the retina, use chandelier lighting and forceps in the other hand, and use true bimanual surgery, employing the cutter as the scissors in such a case.
Dr. Charles: I still use scissors in some cases, especially for the most difficult table top traction retinal detachments. I use the cutter even in those cases for 80% to 90% of the work.
Dr. Murray: We also have the indentation delamination technique, allowing us to go over the membrane, which comes into the port. If you go from the other side, the easiest material to come in is the retina. Not so if you are over the membrane.
Dr. Charles: I call that "fold-back delamination" because it only works if the epiretinal membrane is elastic enough to fold back into the port. Otherwise, you have to do what I call a conformal cutter delamination, the front-on approach. RP
The Ins and Outs of Succeeding With Small-gauge Surgery
Dr. Boyer: Let's turn our attention to factors that can make a difference between success and failure during small-gauge retinal surgery, beginning with illumination. One of the problems we encountered when 25-g technology was introduced was difficulty seeing. Major progress has been made in this area. Do we really need chandeliers now that we have new lighting sources?
CONE OF ILLUMINATION
Dr. Ho: The cone angle of illumination on the original endoilluminator for the 25-g system was narrow. The power on the halogen systems was weak, making it seem like we were operating in the dark. Since then, we have seen three improvements:
- Chandelier systems became easier and more prevalent.
- Endoilluminators became more rigid in the 25-g systems; the cone angles nearly doubled for better illumination.
- The horsepower of the new illuminating boxes — the Accurus High Brightness Xenon Illuminator (Alcon, Fort Worth, Texas) and the Synergetics Photon Illuminator (M.I.S.S. Ophthalmics, Corby, Northamptonshire, UK) — introduce much more light into the eye.
Chandelier lighting systems introduce more light into the eye, allowing for excellent illumination and better outcomes.
I use chandeliers in fewer than 5% of my cases. However, I like to have 25-g plug-in chandeliers or the Dutch Ophthalmic Research Center (DORC) twin-light chandelier system for very complicated cases, in which I need to use a bimanual technique.

One of the problems we encountered when 25-g technology was introduced was difficulty seeing. Major progress has been made in this area. — David S. Boyer, MD |
Dr. Charles: I do not use the chandelier systems for bimanual surgery. Certainly, xenon sources, plus the secondgeneration endoilluminator, at 78°, provide an effective divergence angle for facilitating a view of the vitreous. Anything wider than that would be like driving with your high beams turned on in a fog. It makes it more difficult to see transparent vitreous, and more difficult to see the internal limiting membrane (ILM).
I like the divergence angle of the standard endoilluminator in the pack, which allows me to see the periphery so that I can address every horseshoe tear, pseudophakic retinal detachment and small breaks. The endoilluminator also allows me to see vitreous, ILM and transparent epiretinal membranes. I use the endoilluminator for everything.
Regarding the xenon sources, I offer one word of caution: The brightness of the bulbs diminishes over time, putting your patient at risk for phototoxicity when you replace a spent bulb with a new one but don't change the setting.

The lack of availability of [indocyanine green] really has taken the training wheels away and allowed us to become better surgeons. — Allen C. Ho, MD, FACS |
STAINING ALTERNATIVES
Dr. Boyer: Does anyone use triamcinolone acetonide (Kenalog, Bristol-Myers Squibb) or any other adjunct now that indocyanine green (ICG) is not available?
Dr. Murray: Triamcinolone acetonide is an excellent teaching tool for our fellows, helping them understand the relationship between hyaloid and vitreous. However, I have found it is not uniquely beneficial for peeling ILM, the tissue management plan of most interest to me. Trypan blue also seems to have limited potential in ILM surgery, preferentially staining epiretinal membrane, but not in a significantly advantageous way.
Dr. Ho: I agree but would say it another way: The lack of availability of ICG really has taken the training wheels away and allowed us to become better surgeons. If ICG were made available again, we might use it on ILM only in recurrent cases. Triamcinolone acetonide is not a good stain for ILM, but it can help identify ILM that you have elevated when you are beginning to remove it.
Dr. Charles: If you use the binocular indirect ophthalmomicroscope (BIOM) or any other wide-angle system to perform macular surgery, you will experience a significant loss of both axial and lateral resolution. ICG is used in part because wide-angle systems make viewing the ILM much more difficult.
REMOVING INSTRUMENTS SAFELY
Dr. Boyer: One of the most important aspects of micro-surgery is reducing hypotony while removing instruments. Dr. Charles, when you have finished a case, how do you remove your cannulas?
Dr. Charles: I know that some surgeons prefer to leave the endoilluminator in the cannulas when they pull them out. Others use plugs for a similar purpose. The most important factor is removing the cannulas in a tangential manner, the same way you insert them to set the scleral wound properly.
I leave the IOP at about 20 mm Hg to close the scleral tunnel. I look at the surface of the eye for vitreous wick, which, along with omitting subconjunctival antibiotics, will increase the risk of endophthalmitis.
I agree that triamcinolone acetonide and ICG may be indicated for teaching fellows, but they are not necessarily what we should use.
FOLLOW WOUND DYNAMICS
Dr. Murray: When removing the cannula, you want to follow the dynamics of your wound as you exit. Grasp the conjunctiva around the cannula, stabilize the cannula and remove the cannula in the same track that you entered. As you remove the cannula, make sure you slip the conjunctiva back and close the sclera with your forceps to stabilize the sclera.
I first remove my supranasal and supratemporal sclerotomies, then my infusion cannula over the light pipe so that I don't have an internal ostium. I want to minimize the potential for vitreous wick syndrome, potentially associated with iatrogenic tear or iatrogenic-related endophthalmitis. I evaluate and massage that area to be certain that it is closed.
Dr. Boyer: Do you turn on your infusion when you remove the cannula?
Dr. Murray: No. I like to have the IOP in the eye stabilized, with my plugs in place. I remove the plug with the infusion off, place the light pipe through and then retract over the light pipe. Then I restabilize the IOP with the infusion and remove the second and third ports.

Triamcinolone acetonide is an excellent teaching tool for our fellows, helping them understand the relationship between hyaloid and vitreous. — Timothy G. Murray, MD, FACS |
FOCUS OF INSPECTION
Dr. Boyer: As you inspect the eye at this point, what are you looking for?
Dr. Murray: In most of these cases, if there has been no hemorrhage or significant fluidic alterations in the subconjunctival space, I am hoping to see a closed, frown-shaped ostium. I make sure a significant wick is not coming through that ostium.
I am particularly concerned if anything is coming through the conjunctival space, one of the key signs of displacement. I do not want to see the conjunctiva balloon in that area, nor see the vitreous through the wound. I aim for stable IOP at the end of the case.
CLOSING THE WOUND
Dr. Boyer: Once you have established that the superior two openings are closed and you are down to the last port, do you just pull out the last cannula? How do you check the infusion site wound?
Dr. Murray: Because we manipulate that site the least, that sclerotomy closes readily, even when it is a 20-g wound. I focus primarily on the sites that we have been manipulating, our supranasal and supratemporal sclerotomies.
Dr. Ho: I conduct an end-of-case ritual, starting with use of the indirect wide-angle viewing system with surgeon-controlled depression to inspect 360°. I want to check for peripheral retinal pathology that might need to be addressed.
Next, I try to cover the superior sclerotomies with an internal air fill. I set the infusion pressure at 20 mm Hg, typically lower than the level at which I operate. I use the Boyer technique, removing the cannula with a solid instrument through the cannula system at the angle Dr. Murray described, to minimize distortion of the sclera. I want the scleral fibers to close, so I pull out the cannula over the solid instrument and massage down the tract with a cotton-tipped applicator. This lets the sclera close around the solid instrument — typically the light pipe or the cutter — taking a 0.75 mm 23-g wound down to about 0.6 mm.
We also displace conjunctiva away from the site of the scleral opening. This misalignment of the conjunctival entry in the scleral wound reduces the chance of infection. You will see an oval or slight opening and gape of the scleral wound, which is acceptable.
Dr. Charles: It is important to remember that the elasticity of the sclera is moderate compared to other elastic structures not significant enough to close the wound. I close the wound leaving the IOP pressure on 20 mm Hg and placing pressure over the scleral tunnel that I have created as I pull out the cannula tangential to the sclera.
It is exactly the same as withdrawing a needle from a patient's vein. You press on the wound as you pull the needle out of the vein.
WHEN TO SUTURE
Dr. Ho: It is also important to watch the conjunctival behavior over the scleral wound to determine if it is truly leaking. If you use subconjunctival air or if you do not use air and you see a lot of fluid ballooning up under there, you will know that this is an open system and you will need to massage again.
If I have a leak, I place a single suture without dissection of conjunctiva through the scleral wound. I then use subconjunctival vancomycin for all my cases and a subconjunctival steroid injection to ward off the possibility of infection.
Dr. Murray: I believe in a low threshold for wound suture, especially if you are early in your adoption of this technology. As one gains experience, the need to suture decreases significantly.
Dr. Charles: The suture you choose is important. Formerly, I used to use Vicryl (Novartis Ophthalmics, East Hanover, NJ), but found it causes too much inflammation. I have converted to Biosorb (Imtec Corporation, Ardmore, Okla.), which I highly recommend.

If your patient has a phakic or pseudophakic eye … you cannot achieve minimum inhibitory concentration (MIC) drug levels in the vitreous cavity with any topically applied antibiotic. — Steve Charles, MD |
IMPORTANCE OF SUBCONJ ANTIBIOTICS
Dr. Charles: If your patient has a phakic or pseudophakic eye — that is to say, a two-compartment eye — you cannot achieve minimum inhibitory concentration (MIC) drug levels in the vitreous cavity with any topically applied antibiotic. Our instrumentation enters the vitreous cavity, and that space cannot be reached by topical medications. You must use subconjunctival antibiotics.
Harry W. Flynn, Jr., MD, a world authority on endophthalmitis, uses gentamicin and ceftazidime. I use tobramycin, another aminoglycoside. For broad coverage, I think we should use vancomycin only when it is necessary, per the guidelines of the Centers for Disease Control.
Another important point: Injecting directly over the sclerotomies, as was recommended by some, is a huge mistake. Cases of retinal toxicity have been reported, associated with gentamicin refluxing through the wounds into the eye. Instead, inject in the inferior cul de sac. Another benefit of this approach is that the injection site will not be as visible under the lower lid if some subconjunctival hemorrhaging develops in a patient who is taking clopidogrel bisulfate (Plavix, Bristol-Myers Squibb), aspirin or warfarin sodium (Coumadin, Bristol-Myers Squibb).
Dr. Ho: Besides being aware of the presence of the scleral ostium, the early adopter needs to know that pressure may be low on day 1 post-op and may not normalize for a week. We found this to be the case 7% of the time when comparing a 23-g surgical series of about 120 cases.1 RP
Reference
|
What Surgeons Want in the Future
Dr. Boyer: Many surgeons gave up on 25-g surgery because of the flexibility of the instruments, the poor lighting, the time it required to perform vitrectomy, and difficulty in repairing posterior vitreous detachments. All of you are involved in teaching fellows and residents. What do you tell them now?
NEW ALTERNATIVES
Dr. Charles: I advise them to try the new-generation 25-g and 23-g instruments on alternate cases and compare them.
Clearly, 23-g wounds are more difficult to construct and more likely to leak. You experience more pulsatile vitreoretinal traction because of lower resistance fluidics.
Dr. Murray: I think the revolutionary step forward was 23-g. Once surgeons become comfortable with constructing the wound and exiting the eye, most of them will be thrilled with second-generation 23-g instrumentation.
WHAT WILL THE FUTURE BRING?
Dr. Boyer: What is the future for this technology? What do we not have today that you need or want?
Dr. Charles: As a surgeon who prefers 25-g, I would like to see a flexible laser probe and a fragmenter with a smaller diameter. I do not think bimanual surgery is necessary for most cases. I believe 25-g DSP scissors are excellent, but their cutting ability could be improved slightly.
Dr. Murray: As an advocate for 23-g surgery, I see two holes in coverage that still require 20-g surgery:
■ Complex eyes that have undergone prior vitrectomy
■ Cases in which the conjunctiva needs to be taken down because of a combined scleral buckling procedure.
Otherwise, 23-g will clearly be the procedure of the future for surgical management in most eyes undergoing vitrectomy.
However, I would like to see improvements in the laser delivery probe system and expansion of forceps scissors and picks. Because of the 23-g ostium site, we can use virtually every instrument that we use in 20-g.
I also would like to see a wide-field chandelier system incorporated into the infusion cannula, allowing us to avoid making another ostium. For those complex 23-g cases, I would like to have wide-field illumination, permitting me to perform a bimanual case without secondhand illumination.

We need to improve wound creation, globe stabilization and the appearance of the wound at the end of the case, particularly for 23-g. — Allen C. Ho, MD, FACS |
As we perform complex surgery with the 23-g system, more technologies will help us ensure that the sclerotomy remains closed, without needing to suture it.
I think we will see advances in next-generation platforms, enhancing fluidics and control of the eye. We will be able to control IOP for cases in which we do not need big fluctuations in pressure. This will encourage more stable wound construction.
A DIFFERENT VIEW
Dr. Ho: I see a place for 25-g systems coexisting with 23-g systems. If any system will go out of use, I think it will be the 20-g system.
We need to improve wound creation, globe stabilization and the appearance of the wound at the end of the case, particularly for 23-g. We need a better instrument to fixate the globe.
I also think we could have a better blade to create the 23-g wound. We need to expand our array of instrumentation, including the fragmatome. I really would enjoy having 25-g micro-incision surgery with an illuminated endolaser that provides wide-angle viewing, surgeon-controlled depression and surgeon-controlled laser of peripheral pathology.
In addition, we need better hardware systems to let us inject pharmacologic agents into the eye during surgery. The surgical theater also needs to catch up with our office-based imaging technologies.
Dr. Boyer: These insights will help our colleagues better understand and more effectively use this continually evolving etechnology. RP








