Stemming the Tide of Retinal Degeneration
MICHAEL J. YOUNG, PhD • BUDD TUCKER, PhD • HENRY KLASSEN, MD, PhD
Recent progress in the field of stem-cell biology has created a great deal of enthusiasm for the potential to treat a wide range of previously intractable diseases. This has included, for example, an explosion of research into novel regenerative therapies for many incurable central nervous system disorders, including Huntington’s disease, Parkinson’s disease, and Alzheimer’s disease, as well as various forms of retinal degeneration. Here, we will focus on the latest developments in stem-cell research for the treatment of retinal diseases, such as retinitis pigmentosa (RP) and age-related macular degeneration (AMD). In doing so, we will provide a brief background on the use of stem cells for the preservation and restoration of visual function (via neuroprotection and photoreceptor replacement, respectively) and then focus our attention on 2 recent, instrumental publications1,2 that show promise for patients suffering from blinding diseases.
WHAT IS A STEM CELL?
A stem cell is defined as a cell with an unlimited capacity for self-renewal that also has the ability to generate multiple mature cell types (pluri- or multipotency). Thus, an ideal stem cell should have the ability to generate each of the different cell types of the body an infinite number of times. Although this is believed to be the case for the embryonic stem cells (ES), many of the cells currently used in research, especially in the eye, are much more restricted in terms of cell fate, typically exhibiting the ability to generate multiple cell types only along a specific lineage. For this reason, these cells have been more accurately termed progenitor cells. For instance, skin-derived precursor cells, such as myelinating schwann cells, are destined to become cells of a neural crest lineage, while subgranular zone dentate gyrus progenitors of the hippocampus typically generate mature granule cell interneurons.3-5 Thus, when progenitor cells are transplanted into heterotopic locations, their predetermined phenotypic restriction continues to dominate their eventual fate. For example, we have previously shown that hippocampal progenitor cells transplanted into the vitreous of dystrophic rod-cone syndrome (RCS) rats have the ability to migrate and integrate into each layer of the degenerating retina, but they do not express mature photoreceptor markers and, as such, do not function as mature photoreceptors.6,7 For this reason, more recent studies, such as the 2 that will be discussed here, have utilized either ES cells or precursors already committed to a retinal fate. One of the initial concerns with stem/progenitor cell transplantation, as with transplantation of any allogeneic tissue, was whether these cells would undergo immunologic rejection. These concerns were diminished with early findings suggesting that many locations within the eye, including the anterior chamber, vitreous, and subretinal space, are immuneprivileged sites.8,9 Similarly, it has also been shown that the donor cells in question are also immune privileged, as they fail to induce an immune response within the donor following transplantation.We have shown that transplantation of allogeneic progenitor cells beneath the kidney capsule (a conventional site) of adult mice did not induce a detectable immune reaction.10 Unlike nonprogenitor cell grafts, these cells did not undergo immunologic rejection or necrosis at any of the tested time points. Furthermore, the results between allogeneic and syngeneic progenitor cell transplantations were indistinguishable. In light of these results, the next step was to define the origin and function of these cells.
Michael J. Young, PhD, is an associate scientist and de Gunzburg
Director of the Retinal Transplantation Research Center of the
Schepens Eye Research Institute of the Harvard Medical School in
Boston. Budd Tucker, PhD, is a postdoctoral fellow at Schepens.
Henry Klassen, MD, PhD, is assistant professor of ophthalmology at
the Universiity of California, Irvine. Dr. Young can be reached at via
e-mail at mikey@vision.eri.harvard.edu. None of the authors have a
financial interest in any product mentioned in this article.
ORIGINS AND FUNCTIONS
Embryonic stem cells are isolated during early embryogenesis from the inner cell mass of the blastocyst.

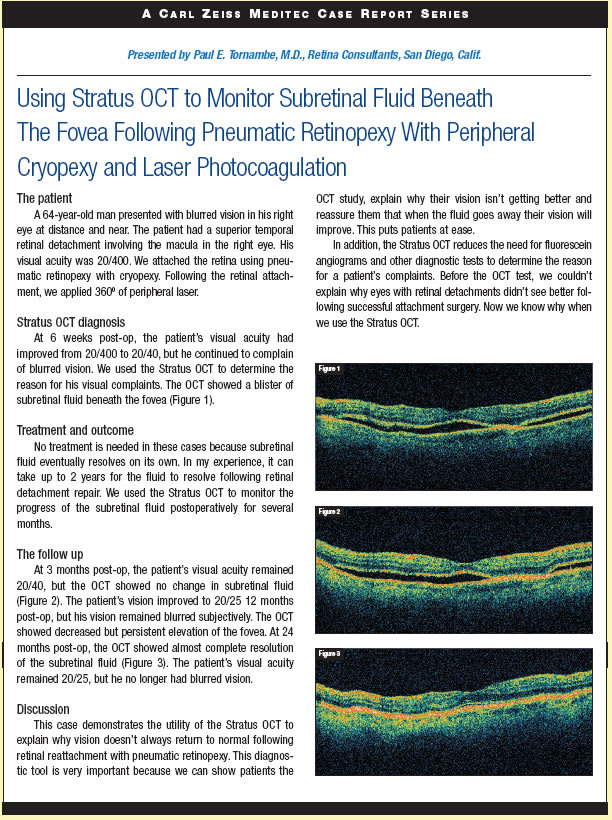
As suggested above, these cells are truly pluripotent and have the ability to generate the various cell types that derive from all 3 embryonic germ layers.11 This means that these cells ultimately have the potential to become any of the different cell types of the mature retina (Figure 1). For instance, Ahmad and colleagues12 have previously shown that mouse ES cells, cultured in the presence of retinoic acid and basic-fibroblast growth factor (bFGF), or co-cultured with postnatal day 1 (P1) retinal neurons, can be induced to express markers such as protein kinase C and metabotrophic glutamine receptor type 6 indicative of a bipolar cell phenotype, or express rhodopsin and arrestin indicative of a photoreceptor cell phenotype.13 Unlike ES cells, retinal progenitor cells (RPCs) have the distinct advantage of being obtained at much later stages in development. For instance, a unique population of RPCs have recently been identified within the ciliary margin zone of the adult mammalian eye.14,15 RPCs, although still considered multipotent, are nervertheless fate restricted and destined to become mature retinal cell types only. Upon isolation and in vitro expansion, these cells form nonadherent cellular aggregates, or neurospheres, which have been shown to express the proliferative marker Ki-67 and the primitive neuroepithelial marker nestin, both of which are characteristic of neural stem cells.16 Treatment of these cells in vitro for 7 days with 10% serum stimulates cellular differentiation and expression of the mature cell markers GFAP, rhodopsin, and MAP2, indicating the presence of glial, rod photoreceptor, and retinal ganglion cells, respectively.16 Upon transplantation into dystrophic eyes, these cells integrate within the existing retinal architecture, adapt the correct photoreceptor morphology, and express the photoreceptor markers rhodopsin and recoverin.7, 16, 17 Furthermore, when transplanted into rhodopsin knockout mice, these cells are associated with improved visual function, as indicated with a behavioral test of photophobia, via either the production of new, or preservation of existing, photoreceptor cells.16
RECENT WORK IN THE FIELD
In light of this work, it is evident that ES cells and RPCs both possess the potential to form the basis of novel experimental therapies for the treatment of retinal degenerations. The recent paper by Lamba and colleagues1 reports the use of human ES cells as a renewable source for the generation of human retinal progenitor cells (hRPCs). In this work, the authors utilized the known molecular biology of early neural histogenesis in vertebrates to develop a combinatorial method for moving ES cells toward the retinal cell fate. The bone morphogenic protein (BMP) and Wnt signaling pathways, known to be involved in forebrain induction, are antagonized by Dickkopf-1 (dkk1) and noggin, respectively. Eye field specification is thought to be induced by insulin-like growth factor-1 (IGF-1). The authors therefore treated ES cell-derived embryoid bodies with dkk1, noggin, and IGF-1, in all possible combinations of these factors. Following differentiation with bFGF, the resulting cells were then characterized with immunocytochemistry, real-time PCR, and calcium imaging of glutamate responses. In addition, explant coculture experiments were also performed. Following 3 days in the retinal progenitor cell induction cocktail and subsequent differentiation in bFGF, a number of retinal specification genes (termed eye field transcription factors [EFTFs] by the authors) were found to be upregulated, including Crx, Rx, and Pax6. Interestingly, all 3 of the candidate factors were needed for this induction to occur, as all combinations of only 2 factors were found to be much less effective. Immunocytochemical and PCR evidence was presented, clearly showing that combined treatment with dkk1, noggin, and IGF-1 causes ES-cell-derived embryoid bodies to develop along a retinal lineage. The expression of a number of EFTFs and retinal-lineage markers were used to compare differentiated human ES (hES) cell-derived RPCs to developing fetal retina (12-week gestation). A remarkably linear relationship between these cells was demonstrated, further arguing that
authentic RPCs were being generated in this study. Although the number of cells expressing photoreceptor markers by these RPCs was exceedingly small, the authors were able to increase this number substantially by coculturing them with explanted degenerating retinas. Rhodopsin, recoverin, and Nrl, all indicative of photoreceptor differentiation, were expressed by the RPCs in this model, and the expression of recoverin was much higher when cocultured with a degenerating retinal explant as compared to a normal control explant. Importantly, the authors also showed that the differentiated RPCs generated with this protocol result in cells that are functionally mature. A subpopulation of cells with neuronal-like morphology was found to respond to glutamate, as measured by calcium imaging. This, together with the expression lineage markers, points to the development of functionally mature amacrine and retinal ganglion cells from hES cell-derived RPCs. This is an important paper, defining a novel means of generating human retinal neurons for transplantation studies by exploiting a potentially inexhaustible cell source, ie, human ES cells. Although several previous studies have made use of ES cells for retinal transplantation studies, none have so elegantly made use of the molecular genetics of embryogenesis to systematically manipulate the fate of human ES cells. Moreover, the analysis of the resultant cell types generated by the treatment regimen is thorough and critical, something not always seen in stem cell studies. A number of interesting issues are raised by this paper. First, it is remarkable that such a high level of differentiation is achieved through the combined treatment with just 3 factors simultaneously. As normal development is obviously much more complicated, both temporally and quantitatively, this points to the potential to dramatically improve the outcome of such studies through controlled delivery of these (and other) factors at specific time points and at optimal concentrations. This bodes well for the future of this field. Second, the fact that the pace of development in this in vitro paradigm was vastly accelerated over normal in vivo developmental time argues that researchers will be able to control the fate of ES cells in culture and generate specific cell types more rapidly than the pace of normal development, again supporting the utility of this approach for the production of cells and tissues for transplantation studies and, ultimately, for the treatment of degenerative diseases.
A MOUSE MODEL
Another recent article that has attracted considerable attention is the study in mice by MacLaren and colleagues,2 where they report that transplantation of postmitotic retinal precursors results in optimal integration of donor cells into the host retina. In this paper, the authors contrast the impressive integration displayed by postmitotic precursor cells with a lack of integration obtained when they grafted actively mitotic cells from the early embryonic retina. This work has succeeded in bringing much needed attention to the field of retinal transplantation and, very importantly, questions current prevailing views regarding the selection of stem and precursor cells for transplantation. Here, we will review the mouse laboratory work described in the paper by MacLaren and colleagues, and then attempt to relate this to the challenges currently facing clinical practitioners in the quest for retinal regenerative therapies. One of the most striking aspects of this paper is the exceptional quality of the microscopy, which reveals in stunning detail the high degree of morphological integration that can be achieved by engrafted cells in the mouse retina. Convincing evidence is presented that once again supports the seminal work of Takahashi and colleagues,18 who first demonstrated the successful integration of grafted progenitor cells into the immature mammalian retina. The authors also beautifully confirm our own findings, in which we demonstrated that cells from the neural retina of newborn mice are capable of integration into the outer nuclear layer of retinal dystrophic recipients, where they differentiate into rods and rescue host light sensitivity.16 Where this paper truly distinguishes itself is in bringing well-deserved attention to the issue of donor cell selection.Whereas previous studies had selected mitotically active cells for transplantation, with the goal of maintaining phenotypic plasticity, the authors of the present study startled the field by reporting similar results following selective transplantation of postmitotic rod precursor cells.
DRAWING THE CORRECT CONCLUSIONS
Some readers have erroneously concluded from this report that there is no longer a reason to contemplate the use of stem or mitotically active progenitor cells for retinal regeneration, something the authors themselves deny.What should be obvious is that an adequate source of transplantable cells is and will remain a central problem for regenerative medicine. To illustrate this point vis-à-vis the current example, in humans, newly committed rod photoreceptor precursors can only be obtained from mid-gestation fetuses, thus presenting a significant dilemma to anyone who might wish to harvest them directly for medicinal purposes.19 Therefore, rather than dispensing with the use of stem cells, the findings of MacLaren imply that the selection for mitotically active cells for purposes of expansion should be followed by a second selection for postmitotic cells for purposes of transplantation. To the extent that their findings with postmitotic precursors can be replicated and extended beyond the mouse model, this would represent a welcomed refinement of present strategy. All the more so when transplanting embryonic stem cells, where proliferation of grafted cells with residual mitotic capacity has bedeviled the field. Selection for mitotically active progenitor cells has thus far provided an effective means of “killing 2 birds with 1 stone,” that is, generating sufficient cells for transplantation while also ensuring that there are sufficient numbers of cells capable of integration with the host retina. This strategy only succeeds because progenitor cells continuously give rise to postmitotic cells, both in culture and following transplantation. This is evidenced by the consistent presence of minority subsets of cells expressing doublecortin and beta-III tubulin within progenitor populations cultured from the brain and retina of a wide range of mammalian species, including mouse,16 pig,17,20 and human.21,22 It stands to reason that, as they differentiate, transplanted progenitor cells will tend to integrate with the host retina at the auspicious moment. Yet, the report of MacLaren and colleagues2 raises the important question of whether we can improve on this method. The answer appears to be a resounding “yes.”We have long questioned why one would attempt to transplant highly plastic ES cells, when tissue-specific progenitors already committed to becoming retinal cells more likely differentiate into the cells of interest (eg, photoreceptors). The very legitimate counterargument points to the need for producing sufficient quantities of donor cells for such work to have practical significance. Thus, the relative strengths of stem-cell isolation and progenitor transplantation have been at odds for some time.We are left with the conclusion that a 2-step approach may ultimately be needed. First, the selection for highly plastic stem cells that can be greatly expanded ex vivo to provide the raw material from which to generate a range of therapeutic modalities. Second, the selection of more specific precursor cells for actual transplantation. This second process might involve several successive steps during which the stem cells undergo sequential commitment, such as occurs during normal development. The final goal is to deliver cells that are restricted to the cellular fate(s) desired, without any tendency to clutter the recipient tissue with unneeded cell types. If postmitotic precursors alone are effective in this role, so much the better.Whether this is accomplished through automated cell sorting, genetic modifications, microscale drug delivery, interactions with biomaterials, or other strategies remains an open question, albeit one of considerable interest. For the practitioner it is clear that a cell-based therapy directed at cell replacement in the retina is still some time away. Nevertheless, the fields of molecular and regenerative medicine are advancing rapidly. For instance, the use of cells as drug-delivery vehicles directed at the neuroprotection of retinal photoreceptors is currently undergoing clinical investigation. Reports of success in reaching this first important milestone might not be long in coming, pointing towards the day when stem-cell technology will be used to repair the diseased retina.
REFERENCES
1. Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor
cells from human embryonic stem cells. Proc Natl Acad Sci U S A.
2006;103:12769-12774.
2. MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of
photoreceptor precursors. Nature. 2006;444:203-207.
3. Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal
neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A.
1999;96:5263-5267.
4. Gross CG.Neurogenesis in the adult brain: death of a dogma. Nat Rev
Neurosci. 2000;1:67-73.
5. McKenzie IA, Biernaskie J, Toma JG, Midha R, Miller FD. Skin-derived precursors
generate myelinating Schwann cells for the injured and dysmyelinated
nervous system. J Neurosci. 2006;26:6651-6660.
6. Young MJ, Ray J, Whiteley SJ, Klassen H, Gage FH. Neuronal differentiation
and morphological integration of hippocampal progenitor cells transplanted to
the retina of immature and mature dystrophic rats. Mol Cell Neurosci.
2000;16:197-205.
7. Van Hoffelen SJ, Young MJ, Shatos MA, Sakaguchi DS. Incorporation of murine
brain progenitor cells into the developing mammalian retina. Invest Ophthalmol
Vis Sci. 2003;44:426-434.
8. Jiang LQ, Jorquera M, Streilein JW. Subretinal space and vitreous cavity as
immunologically privileged sites for retinal allografts. Invest Ophthalmol Vis Sci.
1993;34:3347-3354.
9. Hori J, Joyce NC, Streilein JW. Immune privilege and immunogenicity reside
among different layers of the mouse cornea. Invest Ophthalmol Vis Sci.
2000;41:3032-3042.
10. Hori J, Ng TF, Shatos M, Klassen H, Streilein JW, Young MJ. Neural progenitor
cells lack immunogenicity and resist destruction as allografts. Stem Cells.
2003;21:405-416.
11. Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev
Biol. 2001;17:435-462.
12. Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult
mammalian eye. Biochem Biophys Res Commun. 2000;270:517-521.
13. Zhao X, Liu J, Ahmad I. Differentiation of embryonic stem cells into retinal
neurons. Biochem Biophys Res Commun. 2002;297:177-184.
14. Zhao X, Liu J, Ahmad I. Differentiation of embryonic stem cells to retinal cells
in vitro. Methods Mol Biol. 2006;330:401-416.
15. Tropepe V, Coles BL, Chiasson BJ, et al. Retinal stem cells in the adult mammalian
eye. Science. 2000;287:2032-2036.
16. Klassen HJ, Ng TF, Kurimoto Y, et al. Multipotent retinal progenitors express
developmental markers, differentiate into retinal neurons, and preserve lightmediated
behavior. Invest Ophthalmol Vis Sci. 2004;45:4167-4173.
17. Klassen H, Kiilgaard JF, Zahir T, et al. Progenitor cells from the porcine neural
retina express photoreceptor markers after transplantation to the subretinal
space of allorecipients. Stem Cells. 2007; Epub ahead of print.
18. Takahashi M, Palmer TD, Takahashi J, Gage FH. Widespread integration and
survival of adult-derived neural progenitor cells in the developing optic retina.
Mol Cell Neurosci. 1998;12:340-348.
19. Reh TA. Neurobiology: right timing for retina repair. Nature. 2006; 444:
156-157.
20. Schwartz PH, Nethercott H, Kirov II, Ziaeian B, Young MJ, Klassen H.
Expression of neurodevelopmental markers by cultured porcine neural precursor
cells. Stem Cells. 2005;23:1286-1294.
21. Klassen H, Ziaeian B, Kirov II, Young MJ, Schwartz PH. Isolation of retinal progenitor
cells from post-mortem human tissue and comparison with autologous
brain progenitors. J Neurosci Res. 2004;77:334-343.
22. Schwartz PH, Bryant PJ, Fuja TJ, Su H, O’Dowd DK, Klassen H. Isolation and
characterization of neural progenitor cells from post-mortem human cortex. J
Neurosci Res. 2003;74:838-851.








