Management of Retinal Complications Following Glaucoma Surgery
RICHARD C. LIN, MD, PhD & WILLIAM F. MIELER, MD, FACS, FACR
Glaucoma surgery is generally performed when intraocular pressure (IOP) remains uncontrolled despite maximum tolerated medical therapy and laser. In adults, the most common glaucoma surgeries are trabeculectomy and aqueous humor shunt implantation. Aqueous humor shunts come in various designs: some with valves designed to open only above certain IOPs and others without valves. While these surgeries are usually successful in lowering IOP, they can lead to posterior segment complications. In this review, we discuss the management of vitreoretinal complications following trabeculectomy and aqueous humor shunt implantation. Complications are diverse, ranging from mild, self-limited changes to visually devastating ones. The major complications we review are hypotony, suprachoroidal hemorrhage, endophthalmitis, vitreous hemorrhage, and retinal detachment.
HYPOTONY
Hypotony develops frequently following glaucoma surgery — as often as 42% of the time within 5 years following trabeculectomy with mitomycin C1 and up to 60% of the time after aqueous humor shunts.2 Most hypotony develops because of increased aqueous outflow, through the trabeculectomy filter or the tube. In particular, nonvalved tube implants such as the Baerveldt tube (Advanced Medical Optics, Inc., Santa Ana, Calif) are associated with higher rates of hypotony; indeed, hypotony may occur over 3 times more frequently in Baerveldt tubes (62%) vs Ahmed valves (18% [New World Medical, Inc., Rancho Cucamonga, Calif]).3 In addition, a recent review of hypotony rates with flat anterior chamber describes a range from 16.5% with Baerveldt implants to 3.5% with Ahmed implants.4 Hypotony usually improves without intervention but can be associated with 2 entities: hypotony maculopathy and serous choroidal effusions.
| Richard C. Lin, MD, PhD, is a vitreoretinal fellow at the University of Chicago. William F. Mieler, MD, FACS, FACR, is chair of the Department of Ophthalmology at the Universiity of Chicago. Dr. Mieler can be reached at (773) 702-3838 or by e-mail at wmieler@uchicago.edu. Neither author has any financial interest in any product mentioned in this article. |
Hypotony maculopathy
Hypotony maculopathy is characterized by clinical signs, including macular folds, retinal edema, optic disc edema, and retinal vascular tortuosity, in the setting of decreased IOP (Figures 1a-1c). It is seen in approximately 3.2% of eyes after trabeculectomy with mitomycin C during 1 to 3 year follow-up,5 and up to 8.9% during 5-year follow-up.1 Management should seek to identify and correct the source of increased aqueous outflow. Options for raising IOP include resuturing the scleral flap,6,7 placement of a full-thickness scleral graft,8 autologous blood injection into the bleb,9 application of cryotherapy to the filtration site,10 performing cataract surgery,11 and using a corneal safety-valve incision.12 Restoring IOP with such measures can often reverse the maculopathy, even in long-standing cases (Figure 1d).13 In other cases, distortion of retinal architecture and decreased visual acuity can persist after IOP normalization. In these cases, vitrectomy surgery with perfluorocarbon liquid14 or with peeling of internal limiting membrane followed by gas tamponade15 may improve retinal architecture and function.
Serous choroidal effusion
Choroidal effusion involves the extravasation of fluid from the choroid into the suprachoroidal space. Following aqueous humor shunt implantation, the incidence ranges from about 7% to 33%, depending on the device and series.4 Measures to decrease postoperative hypotony, such as ligation of aqueous humor shunt tubes with polygalactin sutures, help to prevent the development of choroidal effusions. When effusions do develop, most are localized and nonappositional and have no effect on visual acuity. In such cases, the effusions may resolve with either observation or treatment with topical cycloplegic agents and cortico-steroids (Figure 2). To prevent occurrence of suprachoroidal hemorrhage, patients should avoid heavy lifting, bending, straining, or excessive exercise until the choroidals resolve. In more severe cases, choroidal effusions can produce central apposition of retinal tissue or flattening of the anterior chamber. In these eyes, surgical drainage of the choroidal effusions should be considered. In the majority of cases, surgical drainage achieves anatomic resolution of the effusion and may improve vision and resolve hypotony.16
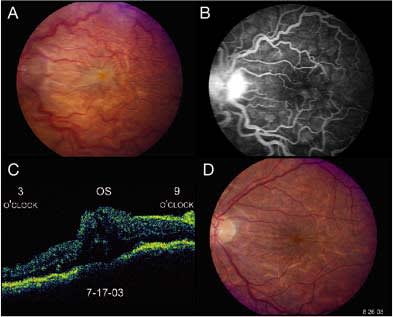
Figure 1 (a) Color photograph OS of a patient 1 week following trabeculectomy surgery. Visual acuity is HM at 3 feet, and the IOP is 3 mm Hg. The disc edema, retinal vascular tortuosity, diffuse retinal folds, and macular edema are all features compatible with hypotony maculopathy. (b) Fluorescein angiogram confirms the clinical findings. (c) OCT shows diffuse macular thickening. (d) Color photograph OS taken 5 weeks later following restoration of normal IOP. The majority of the retinal folds have resolved, along with the macular edema. Vision has improved back to 20/30.
SUPRACHOROIDAL HEMORRHAGE
Suprachoroidal hemorrhage is an arterial bleed into the suprachoroidal space, probably involving rupture of the posterior ciliary artery from the mechanical stress of a choroidal effusion.17 Suprachoroidal hemorrhages may develop in 3% to 8% of eyes following aqueous humor shunt implantation, with an average incidence of 4.2% with various implants.2,4,18 These hemorrhages occur either immediately after surgery or approximately 4 weeks after surgery, resulting from leakage around the tube or from sudden opening of the tube after autolysis of the tube ligature. Appositional suprachoroidal hemorrhages, also known as massive or “kissing” choroidals, can occur following any intraocular surgery but are particularly common following glaucoma surgery. For instance, of all postoperative appositional hemorrhages occurring at a single institution over an 11-year period, 71% of them followed glaucoma surgery or combined glaucoma and cataract surgery.19 Numerous risk factors have been reported for suprachoroidal hemorrhage, including advanced age, atherosclerosis, systemic hypertension, prior ocular surgery, and postoperative hypotony.2,19
As with serous choroidal effusions, small suprachoroidal hemorrhages can be observed (Figure 3). Drainage may be needed for larger hemorrhages, which are associated with central retinal apposition, flattening of the anterior chamber, intolerable pain, retinal detachment, or deteriorating vision. If no concurrent retinal detachment exists, the suprachoroidal hemorrhage can be drained externally through radial sclerotomies, with a limbal infusion line.20 If the retina is detached, a combination of several procedures, including external drainage, vitrectomy surgery, scleral buckling, and injection of a vitreous substitute such as perfluorocarbon, gas, or silicone oil, may help to reattach the retina.20,21 Usually these surgeries are performed after liquefaction of the suprachoroidal hemorrhage, approximately 10 to 14 days after development; surgery should not be delayed if possible, since the presence of apposition for more than 30 days has been associated with a poorer outcome.19 Despite aggressive therapy, appositional suprachoroidal hemorrhages carry a grave prognosis. In most series, approximately one-quarter of eyes end up with no light perception. The prognosis is particularly poor if the hemorrhage has caused incarceration of vitreous or retina in the wound.20
ENDOPHTHALMITIS
Endophthalmitis develops in 1% to 3% of eyes after aqueous humor shunt implantation and 0.3% to 1.8% of eyes after trabeculectomy. This has been of increased concern since the introduction of antifibrotic agents such as mitomycin C, which can lead to more thin-walled, avascular, and leaky blebs (Figure 4). The incidence of late blebitis following trabeculectomy with antifibrotic agents has been reported to be 5%.22 Some studies have suggested that the use of mitomycin C increases the incidence of endophthalmitis23, while others have not found such a trend,1 but no studies have been sufficiently powered to demonstrate a statistically significant increase.24 Early infections occurring within the first month of surgery are thought to be secondary to intraoperative contamination. Late-onset endophthalmitis can occur years after glaucoma surgery and probably arises from transconjunctival spread of bacteria through a bleb leak or an exposed tube. Thus, part of the management of bleb-associated endophthalmitis should be repair of a bleb leak if present. Similarly, if a glaucoma tube is eroding through the overlying conjunctiva, it should be covered with a scleral or pericardial patch graft, and if endophthalmitis occurs in the setting of an eroded or exposed tube, the aqueous humor shunt device should be removed. If the tube is not exposed, it may not be necessary to remove the device.2,4

Figure 2. Serous choroidal detachment in a patient with IOP of 4 mm Hg. This was conservatively managed with observation, and the serous detachment spontaneously resolved following restoration of normal IOP.
More controversial is the role of vitreous biopsy with intravitreal antibiotic injection (tap and inject) vs pars plana vitrectomy (PPV) in the management of endophthalmitis following glaucoma surgery. Because the endophthalmitis vitrectomy study only included patients who developed endophthalmitis within 6 weeks of cataract surgery or secondary intraocular lens implantation, its recommendations cannot necessarily be applied to endophthalmitis following glaucoma surgery. In addition, bacterial strains causing endophthalmitis after both trabeculectomies and aqueous humor shunts are more virulent than those causing postcataract endophthalmitis, with an especially high percentage of cases caused by Streptococcus species.25,26,27,28 It has been suggested that prompt vitrectomy is more beneficial than tap and inject in cases of bleb-associated endophthalmitis, even when presenting vision is better than hand motions.28 However, in the absence of large prospective studies, this idea remains controversial. Despite treatment with either vitrectomy or tap and inject, eyes that develop endophthalmitis after glaucoma surgery tend to have a worse prognosis than those that develop endophthalmitis after cataract surgery.

Figure 3. Suprachoroidal hemorrhage occurred 5 days following glaucoma filtration surgery. It was managed conservatively with observation. The hemorrhage resolved over a 3-week time frame, leaving behind moderate retinal pigment mottling.
VITREOUS HEMORRHAGE
Vitreous hemorrhage occurs in 5% of eyes after aqueous humor shunt implantation.29 Sources of blood include breakthrough bleeding from a suprachoroidal hemorrhage, a deep scleral suture, a retinal break, or a pars plana wound in eyes with pars plana tube insertion.30 In aphakic eyes, additional etiologies include hyphema, an aqueous humor shunt entrance wound, iris rubeosis, and iris-tube contact. Similar etiologies of vitreous hemorrhage can occur after trabeculectomy. Hyphema and vitreous hemorrhage have even been reported after autologous blood injection into the bleb after trabeculectomy.31 Management for vitreous hemorrhage involves identifying the source of the hemorrhage and excluding associated pathology. Observation for spontaneous clearing is appropriate for isolated vitreous hemorrhage. PPV may be needed in the setting of coexisting ocular pathology, eg, in the presence of a rhegmatogenous retinal detachment or suprachoroidal hemorrhage. Occasionally vitreous hemorrhage alone can elevate IOP by compromising aqueous outflow through the tube shunt or trabeculectomy site. This is another indication for PPV.
RETINAL DETACHMENT
Rhegmatogenous retinal detachment occurs in approximately 5% of eyes after aqueous humor shunt implantation, with most occurring within 4 months of surgery.32 They develop in isolation or in conjunction with areas of underlying retinal pathology, including choroidal effusion and suprachoroidal hemorrhage. Additional predisposing factors include lattice degeneration, chorioretinal scar, trauma, uveitis, vitreous incarceration, scleral perforation, and retinal dialysis after pars plana tube placement. Retinal detachment after appositional choroidal effusion or suprachoroidal hemorrhage may be complicated by retinal adhesion. Treatment of retinal detachment after glaucoma surgery involves retinal reattachment surgery and treatment of coexisting pathology. Meticulous opening and closing of conjunctiva are required to prevent bleb leaks and scarring. Care must also be taken during scleral buckling to avoid displacement of aqueous humor shunts. Eyes with retinal detachments following aqueous humor shunt implantation have a very guarded prognosis; in one series of 16 eyes with detachments and Molteno implants (Molteno Ophthalmic Ltd., Dunedin, New Zealand), 7 became phthisical or were enucleated.32 Finally, silicone oil should be used with judiciousness after aqueous humor shunt placement, as migration of oil into the subconjunctival space through both valved and nonvalved devices has been reported.33,34,35

Figure 4. Endophthalmitis developed in this patient 2 months following glaucoma filtration surgery with mitomycin C. The patient was treated with pars plana vitrectomy surgery and intravitreal antibiotics. The infection was suppressed, though visual recovery was very limited at HM at 2 feet.
CONCLUSION
Vitreoretinal complications are fairly common after both trabeculectomy and aqueous humor shunt implantation. The complications discussed can occur singly or in combination. Risk factors for their development include advanced age, systemic vascular disease, and postoperative hypotony. Complications may be more frequent with nonvalved drainage devices such as the Baerveldt tube, but the risk can be lessened by taking measures to decrease postoperative hypotony, such as ligation of the tube with an absorbable suture. In most cases, retinal complications are self-limited and require only observation. However, particularly in cases of massive suprachoroidal hemorrhage and endophthalmitis, visual outcomes are often extremely guarded despite aggressive surgical intervention and anatomic stabilization. RP
REFERENCES
1. Bindlish R, Condon GP, Schlosser JD, et al. Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology. 2002; 109:1336–41.
2. Wirostko WJ, Mieler WF, Levin DS, et al. Hypotony and retinal complications after aqueous humor shunt implantation: the 1999 Dohlman Lecture. Int Ophthalmol Clin. 2000; 40:1–12.
3. Mieler WF, Law SK, Kalenak JW, et al. Hypotony and retinal complications following Ahmed glaucoma valve implantation. In: Stirpe M (ed.). Anterior and Posterior Segment Surgery: Mutual Problems and Common Interests. New York: Ophthalmic Communications Society, Inc, 1998:282–288.
4. Nguyen QH. Avoiding and managing complications of glaucoma drainage implants. Curr Opin Opthalmol. 2004; 15:147–150.
5. Cheung JC, Wright MM, Murali S, Pederson JE. Intermediate-term outcome of variable dose mitomycin C filtering surgery. Ophthalmology. 1997;104:143–149.
6. Cohen SM, Flynn HW JR, Palmberg PF, et al. Treatment of hypotony maculopathy after filtering surgery with mitomycin C. Incidence and treatment. Ophthalmic Surg Lasers. 1995;26:435–441.
7. Schwartz GF, Robin AL, Wilson RP, et al. Resuturing the scleral flap leads to resolution of hypotony maculopathy. J Glaucoma. 1996;5:246–251.
8. Kosmin AS, Wishart PK. A full-thickness scleral graft for the surgical management of a late filtration bleb leak. Ophthalmic Surg Lasers. 1997;28:461–468.
9. Wise JB. Treatment of chronic postfiltration hypotony by intrableb injection of autologous blood. Arch Ophthalmol. 1993;111:827–830.
10. Costa VP, Wilson RP, Moster MR, et al. Hypotony maculopathy following topical mitomycin C in glaucoma filtration surgery. Ophthalmic Surg. 1993;24:389–394.
11. Sibayan SA, Igarashi S, Kasahara N, et al. Cataract extraction as a means of treating post-filtration hypotony maculopathy. Ophthalmic Surg Lasers. 1997;28:241–243.
12. Suner IJ, Greenfield DS, Miller MP, et al. Hypotony maculopathy after filtering surgery with mitomycin C. Incidence and treatment. Ophthalmology. 1997;104:207–214.
13. Oyakhire JO and Moroi SE. Clinical and anatomical reversal of long-term hypotony maculopathy. Am J Ophthalmol. 2004;137:953–955.
14. Duker JS, Schuman JS. Successful surgical treatment of hypotony maculopathy following trabeculectomy with topical mitomycin C. Ophthalmic Surg. 1994;25:463–465.
15. Benson SE, Barton K, Gregor ZJ. Vitrectomy for a persisting macular fold in a case of resolved hypotony maculopathy. Am J Ophthalmol. 2004:138:487–489.
16. WuDunn D, Ryser D, Cantor LB. Surgical drainage of choroidal effusions following glaucoma surgery. J Glaucoma. 2005;14:103–108.
17. Wolter JR, Garfinkel RA. Cilioretinal effusion as precursor of suprachoroidal hemorrhage: a pathological study. Ophthalmic Surg. 198;19:344–349.
18. Hong CH, Arosemena A, Zurakowski D, Ayyala RS. Glaucoma drainage devices: a systematic literature review and current controversies. Surv Ophthalmol. 2005;50:48–60.
19. Moshfeghi DM, Kim BY, Kaiser PK, et al. Appositional suprachoroidal hemorrhage: a case-control study. Am J Ophthalmol. 2004;138:959–963.
20. Wirostko WJ, Han DP, Mieler WF, et al. Suprachoroidal hemorrhage: outcome of surgical management according to hemorrhage severity. Ophthalmology. 1998;105:2271–2275.
21. Feretis E, Mourtzoukos S, Mangouritsas G, et al. Secondary management and outcome of massive suprachoroidal hemorrhage. Eur J Ophthalmol. 2006;16:835–840.
22. Parrish R, Minckler D. “Late endophthalmitis”—filtering surgery time bomb? Ophthalmology. 1996;103:1167–1168.
23. Greenfield DS, Suner IJ, Miller MP, et al. Endophthalmitis after filtering surgery with mitomycin. Arch Ophthalmol. 1996;114:943–949.
24. Wilkins M, Indar A, Wormald R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev 2005;19:CD002897.
25. Al-Torbak AA, Al-Shahwan S, Al-Jadaan I, Al-Hommadi A, Edward DP. Endophthalmitis associated with the Ahmed glaucoma valve implant. Br J Ophthalmol. 2005;89:454–458.
26. Song A, Scott IU, Flynn HW Jr, Budenz DL. Delayed-onset bleb-associated endophthalmitis: clinical features and visual acuity outcomes. Ophthalmology. 2002;109:985–991.
27. Gedde SJ, Scott IU, Tabandeh H, Luu KK, Budenz DL, Greenfield DS, Flynn HW Jr. Late endophthalmitis associated with glaucoma drainage implants. Ophthalmology. 2001;108:1323–1327.
28. Busbee BG, Recchia FM, Kaiser R, Nagra P, Rosenblatt B, Pearlman RB. Bleb-associated endophthalmitis: clinical characteristics and visual outcomes. Ophthalmology. 2004;111:1495–1503.
29. Law SK, Kalenak JW, Connor TB, et al. Retinal complications after aqueous-shunt surgical procedures for glaucoma. Arch Ophthalmol. 1996;114:1473–1480.
30. Kayak S, Temin NF, Dura I, Bark AT, and Saatchi AO, Solve MF. Pars plana vitrectomy with pars plana tube implantation in eyes with intractable glaucoma. Br J Ophthalmol. 1998;82:1377–1382.
31. Flynn WJ, Rosen WJ, Campbell DG. Delayed hyphema and intravitreal blood following intrableb autologous blood injection after trabeculectomy. Am J Ophthalmol. 1997;124:115–116.
32. Waterhouse WJ, Lloyd MA, Duel PU, et al. Rhegmatogenous retinal detachment after Molteno glaucoma implant surgery. Ophthalmology. 1994;101:665–671.
33. Freiberg TR, Famous MM. Migration of intravitreal silicone oil through a Baerveldt tube into the subconjunctival space. Semin Ophthalmol. 2004;19:107–108.
34. Nazmi PP, Chon LP, Varna R, Burns tine MA. Migration of intraocular silicone oil into the subconjunctival space and orbit through an Ahmed glaucoma valve. Am J Ophthalmol. 2001;132:929–931.
35. Hung SM, Min JP. Subconjunctival silicone oil drainage through the Molteno implant. Korean J Ophthalmol. 1998;12:73–75.








