Endophthalmitis Prophylaxis for Intravitreal Injections
RANDALL J. OLSON, MD
The change that has occurred in retinal practices with intravitreal injections is truly dramatic and likely only to increase. The most feared complication of these intravitreal injections is endophthalmitis (Figure 1), and, while the incidence may be less than 1 per 1000, the number of injections that may occur over the lifetime of a patient is such that this is a real concern. With a potential of millions of injections per year, this is a growing public health problem, so anything that can be done to prevent endophthalmitis would be an important step.
EXTRAPOLATING FROM CATARACT DATA
There is very little information about prophylaxis in association with intravitreal injections; however, a growing body of literature exists in regard to cataract surgery. It is clear that prophylaxis in cataract surgery is twofold, with the first part being minimizing the bacterial load on the surface of the eye and the second part attaining sufficient antibiotic inside the eye to prevent an infection that otherwise might have occurred. For instance, we showed that the use of topical ciprofloxacin compared to ofloxacin significantly increased the risk of endophthalmitis, as did a leaky wound the day after surgery (44-fold increase) and breaking the capsule or tearing zonules.2,3
Intravitreal injections, however, are different in regard to prevention in several important ways. While the topical fourth-generation fluoroquinolones currently available do provide a therapeutic level of antibiotics in the anterior chamber, such is simply not the case in regard to the vitreous body, where antibiotic levels are negligible. Therefore, the entire battle in regard to retinal procedures has to be on the surface of the eye.
| Randall J. Olson, MD, is the John A. Moran Presidential Chair, Ophthalmology and Visual Sciences, and chief executive officer of the John A. Moran Eye Center at the University of Utah School of Medicine in Salt Lake City. Dr. Olson is a consultant for Allergan. |
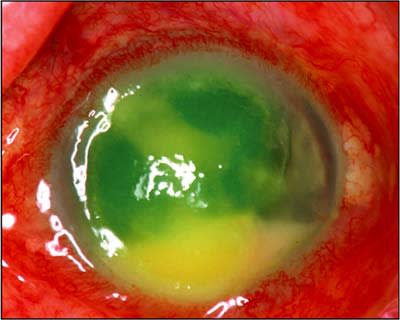
Figure 1. Eye afflicted with endophthalmitis.
THE GENESIS OF ENDOPHTHALMITIS
Just how does endophthalmitis get started after intravitreal injections? Due to the small size of the injection needle, there is never going to be a situation where negative pressure inside the eye will suck contaminated surface material into the eye, as with leaking clear corneal wounds. Furthermore, the small conjunctival puncture wound would seal itself quite rapidly. While such a scenario may occur if the injection site through the conjunctiva and into the sclera is absolutely lined up where postinjection contamination may occur, infection is unlikely even in that event that there is contamination. The problem is completely avoidable by moving the conjunctiva prior to injection so that the 2 openings never line up.
It is almost certain that the problem in regard to endophthalmitis with intravitreal injections is the bore of the needle potentially picking up bacteria from the contaminated conjunctiva. Then, as the material is injected, those bacteria are also literally injected into the vitreous. The problem is that, while it takes a fairly large inoculum to routinely cause endophthalmitis in the anterior chamber, such is not the case for the vitreous. Where thousands of bacteria are necessary in the front of the eye with an intact capsule to produce endophthalmitis, it takes only a few to get the job done in the vitreous. So the strategy must be to minimize the bacterial load on the surface of the eye prior to injection. It would also be important to minimize the risk of injecting multi-drug-resistant bacteria.
Prophylaxis should always include topical povidone-iodine, which does an excellent job of decreasing the bacterial load. Fortunately, the effect of povidone-iodine and antibiotic are additive. Therefore, appropriate antibiosis is another way to decrease the bacterial load.
TREATMENT WITH ANTIBIOTICS
Looking at antibiotics, topical fourth-generation fluoroquinolones have been the mainstay of treatment for a host of different reasons. The minimal inhibitory concentrations (MICs) necessary to eradicate the bacteria are favorable and resistance is much less common but, unfortunately, starting to occur.4 The main difference between the 2 antibiotics is that one is 0.5% moxifloxacin without preservative, whereas the other is 0.3% gatifloxacin with benzalkonium chloride (BAK). As far as any clinical evidence that one is superior to the other in cataract prophylaxis, the only large study presented so far looking at the 2 fluoroquinolones found that gatifloxacin 0.3% with BAK had approximately half the incidence of endophthalmitis of 0.5% moxifloxacin. However, the differences were not statistically significant (P=.11).5
From an intravitreal injection standpoint, however, there may be differences that deserve further scrutiny. BAK (found only with 0.3% gatifloxacin) decreases the MIC in virtually all organisms, including resistant organisms.6 This effect has been as much as a 2.0+ log unit reduction, such that organisms that otherwise might be completely resistant to antibiosis are rendered sensitive. This could be an important advantage in regard to minimizing bacterial load of multiple-drug-resistant organisms.
The second effect may be even more important, and that is the rapidity with which organisms are eliminated with the combination of BAK plus antibiotic. Looking at bacterial eradication after 15 minutes of antibiosis, both fourth-generation fluoroquinolones alone (ie, no BAK) had unsubstantial or minimal reduction in the bacterial load. This is consistent with a recent clinical study looking at short-term antibiosis with 0.5% moxifloxacin,7 however, either fourth-generation antibiotic with BAK had about a 5 log unit reduction in 5 to 15 minutes with minimal effect without BAK in in vitro studies.8 For the short-term prophylaxis typical for intravitreal injections, 0.3% gatifloxacin with BAK would appear to have an advantage (Figure 2).
RECOMMENDATIONS
Suggested best practices for reducing the bacterial load prior to an intravitreal injection would be 5 drops of 5% povidone-iodine solution with frequent placement of a topical fourth-generation fluoroquinolone at least 5, and preferably 10, minutes prior to injection. For the reasons mentioned, 0.3% gatifloxacin would appear to have an advantage in eliminating resistant organisms and increasing the speed of kill. Because topical antibiosis is not going to effect endophthalmitis if the vitreous is seeded, long-term use after injection is wasted, so 1 or 2 days postinjection at qid is probably sufficient.

Figure 2. Eye after treatment with antibiotics.
This suggested approach is going to be tested in a series of studies. We hope in the not-too-distant future to have the facts one way or the other, especially regarding our supposition that the needle bore is the culprit in picking up bacteria from the conjunctiva and that BAK is advantageous in minimizing this risk. In the meantime, the evidence we have presented is supportive of an approach for prophylaxis of endophthalmitis associated with intravitreal injections. RP
REFERENCES
1. Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intravitreous injections. Retina. 2004;24(Suppl 5):S3-S19.
2. Jensen MK, Fiscella RG, Crandall AS, et al. A retrospective study of endophthalmitis rates comparing quinolone antibiotics. Am J Ophthalmol. 2005;139:141–148.
3. Wallin T, Parker J, Jin Y, Kefalopoulos G, Olson RJ. Cohort study of 27 cases of endophthalmitis at a single institution. J Cataract Refract Surg. 2005;31:735–741.
4. Deramo VA, Lai JC, Fastenberg DM, Udell IJ. Acute endophthalmitis in eyes treated prophylactically with gatifloxacin and moxifloxacin. Am J Ophthalmol. 2006;142:721–725.
5. Moshirfar M, Feiz V, Vitale AT, Wegelin JA, Basavanthappa S, Wolsey DH. Endophthalmitis after uncomplicated cataract surgery with the use of fourth-generation fluoroquinolones: a retrospective observational case series. Ophthalmology. 2006; Epub ahead of print.
6. Novosad BN, Callegan MC. Killing of streptococcus pneumoniae and haemophilus influenzae ocular isolates by fourth-generation fluoroquinolones. Paper presented at: Annual meeting of the Association for Research in Vision and Ophthalmology; April 30, 2006 – May 4, 2006; Fort Lauderdale, Fla.
7. Ta C, Dhatt H, Paterno J, et al. Prospective comparison of one-day vs one-hour preoperative moxifloxacin prophylaxis for intraocular surgeries. Annual meeting of the American Academy of Ophthalmology; November 11–14, 2006; Las Vegas, Nev.
8. Callegan MC, Novosad BN. Efficacy of fourth-generation fluoroquinolones against gram-positive species commonly involved in ocular inflections. Presented at: Annual meeting of the Association for Research in Vision and Ophthalmology; April 30-May 4, 2006; Fort Lauderdale, Fla.








