PEER
REVIEWED
Update
on Posterior Segment Emergencies
THUCANH
T. HO, MD, & MATHEW W. MACCUMBER, MD, PHD
Ophthalmologists, particularly retinal specialists,
are faced with a variety of vision-threatening emergencies that must be handled
in an expeditious but careful
manner in order to optimize visual outcome. In
1998, the field was covered in a text edited by one of us entitled Management
of Ocular Injuries and Emergencies (MacCumber MW, ed. Philadelphia, Pa: Lippincott,
Williams & Wilkins, 1998). This update highlights several areas where ocular
emergency management has since changed, focusing on the recent increase in blunt
trauma due to paintball war games and airbags, imaging, management of open-globe
injuries, medical management of choroidal neovascularization (CNV) and retinal vascular
occlusion, and endophthalmitis.
|
|
|
|
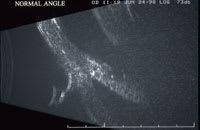
| Figure 1. High frequency ultrasound image of a normal eye (top) and of the opposite eye that developed angle recession after blunt trauma (bottom). |
BLUNT AND PENETRATING TRAUMA
Worldwide, approximately 55 million eye injuries are believed to occur yearly, leaving 1.6 million eyes blind.1 Globally, there are approximately 200000 open-globe injuries each year with an estimate of 3.5/100000/year of perforating eye injuries.1
Schrader2 looked at 1026 open-globe injuries occurring between 1981 and 1999 and found that the incidence of injuries sustained at work and in traffic accidents decreased over time, with an associated decreased incidence of blindness and enucleations. Unfortunately, the incidence of injuries related to hobbies increased during the same period. One such recreation-related ocular injury that has become prevalent in recent years is seen with paintball war games.
Paintball Injuries
Conn et al3 analyzed the paintball-related injury data from 1997 to 2001 from the National Electronic Injury Surveillance System, a data bank of injuries that are reported from a probability sampling of 100 US hospital emergency departments, and they found an average annual rate of 4.5 injuries per 10000 participants, with the number treated in hospital emergency departments more than 3 times higher in 2001 than in 1997. The eye was found to be the most commonly affected body part (42.7%). At least 85% of those suffering from eye injuries were either not wearing eye protection or had eye protection that was temporarily removed due to fogging or paint splattering.
Paintball guns were originally developed for use by foresters to mark trees for harvest. In the early 1980s, they were adapted for war games and have increased in popularity over the past 2 decades. Paintball pellets have a face-diameter of 17 mm (approximately the diameter of a dime) and are 3.5-g gelatin capsules filled with nontoxic, water-soluble paint designed to rupture on contact. A paint ball travels at a velocity of 300 feet per second when discharged from CO2-powered guns and can cause devastating blunt ocular damage through coup and contrecoup effects and anterior-posterior compression with equatorial expansion. The high velocity, small size, and high mass of paintballs can result in severe trauma. Anterior segment complications include lens trauma, angle recession, iridodiaylsis, and, most commonly, hyphema. Posterior segment complications include choroidal rupture, retinal dialysis and detachments, open globe injury, and, most commonly, commotio retinae and vitreous hemorrhage.4 Listman5 found that 43% of patients had resulting vision of 20/200 or worse.
The American Society for Testing and Materials (ASTM) issued the Standard Practice for Paintball Field Operations, specifying minimum safety requirements, which are used voluntarily by most commercial facilities. The ASTM also issued standard specifications for eye protection devices intended to minimize the risk of eye injuries. These standards have significantly decreased the rate of paintball-related injuries in commercial facilities. However, injuries occurring after 1995 were almost 6 times more likely to occur during noncommercial games than those sustained before 1995. This is due to increased availability of paintball equipment, underutilization of eye protection, and no supervision in "backyard" games. Fineman4 recommended restriction on sales of paintball equipment, public education, and increased adult supervision.
Airbag Injuries
|
|

|
|

|
| Figure 2. This 14 year old boy was hit in the left eye by a baseball. (Top) Clinical photo 4 days after the injury, VA 20/200. (2nd from top) OCT showed inner lamellar macular hole and nasal subretinal fluid. (2nd from bottom) Clinical photo after 6 months observation revealed the development of epiretinal membrane, VA 20/25. (Bottom) OCT showed the inner lamellar macular hole with resolution of the subretinal fluid. |
Overall, the most common sources of eye injuries during motor vehicle accidents (MVAs) are windshields, frontal airbags, steering wheels, and flying glass. Frontal airbags were mandated starting in 1993 and the combined use of seat belts and frontal airbags decreased the rate of MVA-related mortality by 45% and severe injury by 50%.6 Before 1993, windshields were the main source of eye injuries, but this was surpassed by frontal airbags with their availability.7 The major mechanisms of frontal airbag-related eye injuries include contusion with transfer of energy from the vehicle to the passenger, lacerations, and chemical burns from sodium hydroxide release. McGwin et al7 looked at the 1988 to 2001 National Automotive Sampling System Crashworthiness Data System, finding an eye injury rate of 18 in 1000 occupants, with eyelid injuries and corneal abrasions accounting for 94% of such injuries. Frontal airbag deployment was associated with a twofold increased risk of eye injuries compared to the twofold decrease risk associated with seat belt use. Using the same database, Duma et al8 compared the MVA-related eye injury rate with full-powered airbag deployment (1993-1997) vs depowered airbag deployment (1998-2001) and found a 3.7% and 1.7% rate of eye injuries, respectively. Pearlman et al6 reviewed the literature published between 1991 and 2000 on 101 patients who sustained MVA-related eye injuries. They reported that 12.5% of injuries were bilateral and 4.2% were unilateral open-globe injuries. Vitreous or retinal hemorrhage was seen in 9.9% and retinal tears or detachment was seen in 5.7% of cases. The median visual outcome was 20/40.6 Lehto et al9 reviewed the literature published between 1991 and 2001 on 121 patients and estimated the risk of eye injury to be 2.5% and serious eye injury to be 0.4%. They also found a threefold increased risk of open-globe injuries in eyeglass wearers.
Imaging Studies
Eyes with possible open-globe injuries, especially when intraocular
foreign bodies (IOFBs) are suspected, usually require imaging to confirm the diagnosis
and determine the presence, location, and number of IOFBs. Noncontrast computerized
tomography (CT) scanning has replaced conventional radiography as the diagnostic
study of choice for all forms of ocular trauma. CT provides much more reliable information
on size, shape, and localization of the foreign body, whether in the anterior or
the posterior segment.10-13
Drawbacks of conventional CT include separate scanning in axial and coronal planes
leading to prolonged scanning time and radiation exposure. Reconstruction is limited
by stair-step artifact, compromising detection and localization of small and multiple
foreign bodies, especially those adjacent to the sclera or optic nerve. Volume averaging
also hinders detection and localization of small and multiple foreign bodies.14
Current spiral CT scanning with both 1-mm and
3-mm cuts can detect metallic
IOFB to as small as 0.5 mm with nearly 100% sensitivity.15
Spiral CT allows continuous scanning in less time with volumetric, overlapping data
acquisition, providing multiplanar reconstruction of high-quality coronal and sagittal
images. Thinner slices mean less volume averaging and better detection and localization
of small and multiple foreign bodies. Size and location of IOFBs detected on helical
CT corresponded with what was found during surgical and clinical follow-up.14
In eyes with small, stable wounds, judicious use of B-scan ultrasonography over closed eyelids has proved useful in detecting retinal detachment (RD), double perforation, and foreign bodies not otherwise seen on CT, such as vegetative IOFBs.16-18 Using high-frequency (50 MHz) sound waves, ultrasound biomicroscopy is able to create high-resolution, 2-D cross-sectional anterior segment images to a depth of 5 mm (Figure 1). It is a useful adjunct in the detection of small foreign bodies, including those of nonmetallic composition, not otherwise detected by CT and/or B-scan.19,20 Magnetic resonance imaging is only occasionally useful in the setting of ocular trauma, such as the detection of radioopaque IOFBs in large, unstable wounds, but its use must be avoided when iron-containing (ie, metallic) IOFBs are suspected.21-27
Optical coherence tomography (OCT) has revolutionized our ability to detect and differentiate blunt injuries involving the macula. For example, inner lamellar holes can be difficult to differentiate from full-thickness macular holes on slit lamp biomicroscopy but can now be readily diagnosed by OCT, thus improving our ability to advise patients on prognosis and management (Figure 2).
Management of Open Globe Injuries
All patients with severe ocular injuries should have initial evaluation to determine primarily whether the globe is open to an extent that requires surgical intervention in the operating room, whether the patient is medically stable for surgery, and whether there is an IOFB to plan the surgical approach (ie, primary vitrectomy for posterior segment IOFB removal).28 The surgeon should take note of certain characteristics of the ocular injury that are of particular prognostic significance: the mechanism of injury, the presenting visual acuity, the presence or absence of relative afferent papillary defect, and the posterior extent of the wound (zone).29 Patients without recent immunization should receive tetanus prophylaxis. In general, patients should be given systemic antibiotics such as a fourth-generation fluoroquinolone and, if necessary, antiemetics. In cases of vegetable matter, coverage against Bacillus species with systemic clindamycin or vancomycin should also be considered. All protruding foreign material should be left undisturbed and a shield should be placed over every eye with a suspected open-globe injury until the time of surgical repair (Figure 3). Preoperative topical medication can be withheld if there is risk of disrupting the wound, especially in the hands of individuals unfamiliar with the management of eye trauma.
|
|
|
Figure 3. Protruding intraocular foreign body should be shielded (eg, with a plastic cup) and removed in a controlled fashion in the operating room |
Traditionally, general anesthesia is utilized in the management of ocular trauma. However, this is contraindicated in high-risk patients and is associated with a longer recovery period. Recently, some surgeons have investigated the use of intravenous sedation with local, or even topical, anesthesia in selected patients. Scott et al30,31 analyzed 220 eyes with open-globe injuries managed at Bascom Palmer Eye Institute in Miami between 1995 and 1999 and 238 cases managed between 2000 and 2003. Sixty-four percent and 59% of these cases, respectively, were performed under intravenous sedation and regional anesthesia, in the form of retrobulbar or peribulbar blocks with augmentation after conjunctival cutdown. Patients managed under local anesthesia and intravenous sedation were more likely to have better presenting visual acuity, IOFBs, and more anteriorly located and shorter wounds. In addition to the well-known complications of retrobulbar blocks, care must be taken to prevent expulsion of intraocular content in the presence of larger wounds. Additionally, Boscia et al32 looked at the use of topical anesthesia and intravenous sedation in 10 open-globe cases and found it to be a reasonable alternative in less severe injuries.
Some controversy still exists in surgical management of severe posterior segment injuries.28 The use of prophylactic intravitreal antibiotics varies, but many retinal surgeons have become more comfortable using such antibiotics in the setting of primary repair and IOFB. Secondary vitrectomy procedures traditionally occurred at 10 to 14 days after injury to allow for reduced choroidal congestion and posterior hyaloid separation; however, technicological improvements can permit vitrectomy sooner and more safely to prevent fibrous ingrowth and proliferative vitreoretinopathy.33 Personally we prefer vitrectomy within 1 week for severe posterior segment trauma unless hemorrhagic choroidal detachment demands further delay. There are advocates for and against prophylactic scleral buckling, but we usually choose to place a prophylactic encircling scleral buckle for more severe injuries, particularly those with retinal detachment and when the crystalline lens is preserved.34
CHOROIDAL NEOVASCULARIZATION
|
|
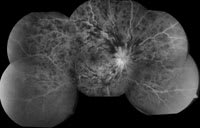
| Figure 4. Montage of a perfused central retinal vein occlusion. |
Although usually not true ophthalmic "emergencies," sudden vision loss due to CNV or retinal vascular occlusion (below) often present in an emergent manner. Progress in the treatment of CNV over the past 10 years has been remarkable. In 1998, the only proven therapy was photocoagulation, which stabilized or improved vision primarily only in selected juxtafoveal and extrafoveal cases. Subsequently, photodynamic therapy (PDT) with verteporfin (Visudyne, Novartis) became available as the first treatment for subfoveal CNV to stabilize and occasionally improve vision.35 Interest in intravitreal triamcinolone acetonide (IVTA, Kenalog, Bristol-Myers Squibb) added to our ability to treat both CNV and macular edema due to a variety of causes.36 Recently, intraocular antivascular endothelial growth factor (VEGF) inhibition has become a reality, initially with pegaptanib (Macugen, OSI),37 then bevacizumab (Avastin, Genentech),38 and now ranibizumab (Lucentis, Genentech).39 The remarkable benefit of ranibizumab has been clear in randomized clinical trials for age-related macular degeneration (AMD).38 We are still learning how to use these therapies alone or in combination for the management of CNV due to different etiologies, including retinovascular emergencies.
Choroidal ruptures are seen in up to 5% to 10% of blunt ocular injuries.40 Unlike the sclera and retina, the retinal pigment epithelium-Bruch's membrane-choriocapillaris complex is relatively inelastic and may break in a concentric pattern around the optic nerve. CNV can arise at the site of choroidal ruptures from 1 month to 4 years after injury.41 Both laser photocoagulation and subretinal surgery have been attempted with limited success. Recently, there have been a few reported cases of choroidal rupture-related CNV responsive to PDT.42-44 Harrisi-Dagher et al43 looked at 5 cases that required an average of 2 treatments and found the absence of or decreased leakage on fluorescein angiogram, with stabilization or improvement in visual acuity in 80% of cases.
Bevacizumab is a humanized monoclonal antibody that inhibits all isoforms of VEGF and is Food and Drug Administration-approved only as an intravenous therapy for metastatic colorectal cancer. There are numerous case reports and case series on the off-label use of intravitreal bevacizumab for CNV secondary to AMD and pathological myopia with dramatic results.45-53 Although, to the best of our knowledge, there are no specific reports on the effect of bevacizumab on choroidal rupture-related CNV, we would expect that it may also have a beneficial effect in these cases.
|
|
|
|
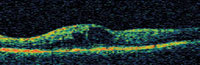
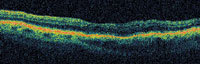
Figure 5. OCT scan of cystic macular edema secondary to BRVO before (top) and 1 week after injection of 1.0 mg bevacizumab (bottom); Snellen visual acuity improved from 20/70 to 20/50. |
RETINAL VASCULAR OCCLUSIONS
Over the past 10 years, there has been substantial improvement in the understanding of the etiology of retinal vascular occlusion, particularly in the younger patient population. Use of birth control pills, dehydration, and embolic events are all potential contributors to retinovascular occlusion in children and young adults. However, no clear etiology is evident by history and review of system in many cases. This has often resulted in an extensive workup that can include MRI/MRA and echocardiography as well as a myriad of blood studies, including complete blood count, folic acid, vitamin B12, antinuclear antibody, rheumatoid factor, erythrocyte sedimentation rate, and lipid profile. In the past, we have been disheartened by the poor yield of these tests; however, each passing year has improved our understanding of the immune and coagulation systems, so that now we can detect a predisposing abnormality in many patients. Newer tests include antiphospholipid antibody profiles, antithrombin III, protein C, protein S, prothrombin, homocysteine, C-reactive protein, factor V Leiden, and mutations in methylene tetrahydrofolate reductase. We hope that we will learn to better target these tests to certain clinical scenarios and perhaps even devise preventive measures.
Progress in the treatment of retinal arterial occlusion has been significantly limited in part due to delay in presentation to the ophthalmologist until after irreversible damage has occurred. Anterior chamber paracentesis is still the only treatment that has improved vision in our hands. However, there have been significant developments in the treatment of retinal vein occlusion (RVO). In the late 1990s, treatment of RVO had been largely limited to photocoagulation. The Central Vein Occlusion Study52 demonstrated no visual acuity benefit from grid laser photocoagulation for macular edema associated with central retina vein occlusion (CRVO, Figure 4) while the Branch Vein Occlusion Study53 demonstrated a treatment benefit with grid laser photocoagulation in some patients with nonischemic branch retinal vein occlusion (BRVO) and less than or equal to 20/40 vision who had not improved after 3 months of observation.
Recently, there is considerable interest in new pharmacologic treatments for RVO and secondary macular edema. Unlike macular laser treatment, these do not require clearing of macular hemorrhages so they can be employed sooner. Surgical and other laser therapies have also been investigated but, in our experience, are less commonly effective. Several small retrospective as well as prospective case series have shown at least a temporary beneficial effect with IVTA in terms of improvement in visual acuity (VA) and reduction in central macular thickness (CMT) on OCT for the treatment of macular edema secondary to BRVO54-60 and CRVO.61-67 Notably, VA has not been shown to correlate with CMT. Ozdek et al61 looked at 11 nonischemic and 11 ischemic CRVO eyes and found at least a 3 line improvement in 82% of the nonischemic group compared to only 18% of the ischemic group. Ramezani et al62 found the therapeutic effects of IVTA 4 mg in CRVO to be greatest in the first 2 months. In a prospective, randomized trial involving 63 eyes with macular edema secondary to diabetic retinopathy (DR) or RVO, Avitabile et al68 compared treatment with IVTA 4 mg, grid laser photocoagulation, or both. At each of the 1-, 3-, and 9-month follow-up visits, VA improved 0.19 to 0.26 logMAR and CMT decreased 29% to 37% in the IVTA alone and combination groups while the VA remained unchanged and CMT decreased only 5% to 16% in the laser group. Hayashi et al69 performed a randomized clinical trial on 60 eyes with BRVO, comparing IVTA 4 mg with 3 repeated retrobulbar injections of 40 mg of triamcinolone acetonide, both groups injected 1 week after grid laser, and found IVTA to be significantly more effective. Based on such results, the National Eye Institute is currently funding the Standard Care vs COrticosteroid for REtinal Vein Occlusion (SCORE) Study, a multicenter, randomized, controlled clinical trial to investigate the safety and efficacy of IVTA for the treatment of macular edema secondary to CRVO and BRVO.
The associated ocular complication rate of IVTA is relatively high when compared with other intravitreal injections. Forty percent develop ocular hypertension with 1% to 2% uncontrolled by medical therapy.70 Cataract extraction is performed within 1 year of treatment in 15% to 20% of injected eyes.70 Because of its ocular safety profile, the use of intravitreal bevacizumab for macular edema secondary to vein occlusions has recently gained popularity (Figure 5).38,71-73 Iturralde et al71 reviewed the results of 16 eyes injected with an average of 2.8 injections on bevacizumab 1.25 mg. Nine patients were previously given IVTA but either failed treatment or developed ocular hypertension. The mean VA improved from a baseline of 20/600 to 20/200 at 1 month and 20/138 at 3 months, and CMT improved from 887 μm to 372 μm at 1 month.
Both IVTA74 and, to a more profound extent, bevacizumab75-79 have also been reported to cause regression of iris neovascularization (NVI), making them much needed additions to our armamentarium for neovascular glaucoma (NVG) management, a condition known for its dismal prognosis. Jonas et al74 treated 14 eyes with NVG (9 diabetics, 5 CRVO) with IVTA 20 mg as the only procedure in 4 eyes, with cryotherapy in 7 eyes, with goniosynechiolysis in 1 eye, and with phacoemulsification in 2 eyes. The degree of NVI decreased from 2.6 (±1.3) to 1.3 (±1.2) relative units post-injection. Both intracameral and intravitreal bevacizumab have been shown to consistently be associated with dramatic regression of NVI within 1 week of treatment with improvement in intraocular pressure control. These case reports suggest that the effect can last for up to 2 months post-injection.75-79
ENDOPHTHALMITIS
|
|
|
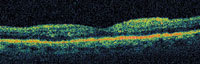
| Figure 6. OCT scan of an eye with diabetic macular edema before (top) and 2 weeks after an intravitreal injection of 4.0 mg trimacinolone acetonide (bottom). There was transient, but marked non-infectious endophthalmitis starting 1 day after injection that cleared over the following week. Vitreous tap revealed no organism growth. Snellen visual acuity improved from 20/70 to 20/50 despite the transient inflammation. |
The explosion in the use of intravitreal injections has generated significant concern about another wave of iatrogenic endophthalmitis. Jager et al80 reviewed the literature from 1966 to 2004, looking at a total of 14 866 intravitreal injections for 4382 eyes and found the overall prevalence of infectious and noninfectious endophthalmitis to be 0.3% per injection and 0.9% per eye. The rate of infectious endophthalmitis was 0.2% per injection and 0.5% per eye. Regarding 1739 IVTA injections, the overall prevalence of endophthalmitis was 1.4% per injection and 0.6% per injection for infectious endophthalmitis. IVTA injections can cause a noninfectious and self-limited endophthalmitis (Figure 6) that may be an immune response to endotoxin or preservatives in certain IVTA preparations; this entity usually has milder inflammation, occurs sooner (hours to days after injection), and is painless. Intravitreal pegaptanib has a reported infectious endophthalmitis risk of 0.16% per injection; the risk is lower with the stricter revised injection protocol.37,81 Derived in large part from data from the VEGF Inhibition Study in Ocular Neovascularization (VISION) trial,37,81 we have learned that proper sterile technique, including the use of a lid speculum and 5% povidone-iodine, can keep the endophthalmitis risk acceptably low.82 Management would usually involve vitreous tap and injection of intravitreal antibiotics if infectious endophthalmitis is suspected.
Taban et al83 performed a review of the literature from 1963 to 2003, pooling 3 140 650 cataract extractions and found the overall incidence of postcataract endophthalmitis to be 0.128%. Incision type was implicated in the increased incidence of postcataract endophthalmitis in recent years, with 0.189% incidence for clear corneal incisions vs 0.074% incidence for scleral incisions between 1992 and 2003. Using OCT, McDonnell et al84 demonstrated that transient postoperative IOP reduction causes the clear cornea cataract wound edge to gape, starting at the internal aspect of the wound. Ciulla et al85 reviewed the literature from 1966 to 2000 on endopthalmitis prophalaxis for cataract surgery and found that 5% povidone-iodine had a particularly significant inhibitory effect.
The results of the Endophthalmitis Vitrectomy Study (EVS)86 published in 1995 showed no benefit of vitrectomy and intravitreal antibiotics over vitreous tap and intravitreal antibiotics for postcataract endophthalmitis patients with better than light perception vision. With modern vitrectomy technology, including panoramic viewing, better instrumentation, and silicone oil, Kuhn and Gini87 advocated the early use of complete vitrectomy for patients with no retinal details or a poor red reflex on ophthalmoscopy, regardless of visual acuity. They reported a consecutive series of 47 eyes that underwent vitrectomy for infectious endopthalmitis, 91% of which recovered at least 20/40 vision compared to 53% in the EVS.
Ng et al88 in the Endopthalmitis Population Study of Western Australia (EPSWA) reviewed 213 cases of endophthalmitis between 1980 and 2000 and found that management involved increased use of intravitreal antibiotics and decreased use of subconjuctival and intravenous antibiotics. Other studies confirm that subconjunctival antibiotics are probably not required in the treatment of infectious endophthalmitis.89,90
CONCLUSIONS
The spectrum and management of posterior segment emergencies continues to evolve. Recent advances have resulted in better diagnosis and management of several posterior segment emergencies, including posterior segment trauma, retinal vascular occlusions, and endophthalmitis. Improved imaging modalities, vitrectomy technology, and anesthesia approaches have permitted more expeditious care of the traumatized eye.
Pharmaceutical developments have added to the outpatient armamentarium; in particular, anti-VEGF therapies have revolutionalized not only how we manage exudative AMD, but also other causes of CNV, retinal vein occlusions, and neovascular glaucoma. Endophthalmitis continues to be a concern in the era of clear corneal cataract surgery and frequent use of intravitreal injections.
REFERENCES
1. Negrel AD, Thylefors B. The global impact of eye injuries. Ophthalmic Epidemiol. 1998;5:143-169.
2. Schrader WF. Open globe injuries: epidemiological study of two eye clinics in germany, 1981-1999. Croat Med J. 2004;45:268-274.
3. Conn JM, Annest JL, Gilchrist J, Ryan GW. Injuries from paintball game related activities in the united states, 1997-2001. Inj Prev. 2004;10:139-143.
4. Fineman MS. Ocular paintball injuries. Curr Opin Ophthalmol. 2001;12:186-190.
5. Listman DA. Paintball injuries in children: more than meets the eye. Pediatrics. 2004;113:e15-18.
6. Pearlman JA, Au Eong KG, Kuhn F, Pieramici DJ. Airbags and eye injuries: epidemiology, spectrum of injury, and analysis of risk factors. Surv Ophthalmol. 2001;46:234-242.
7. McGwin G Jr, Owsley C. Risk factors for motor vehicle collision-related eye injuries. Arch Ophthalmol. 2005;123:89-95.
8. Duma SM, Rath AL, Jernigan MV, Stitzel JD, Herring IP. The effects of depowered airbags on eye injuries in frontal automobile crashes. Am J Emerg Med. 2005;23:13-
9. Lehto KS, Sulander PO, Tervo TM. Do motor vehicle airbags increase risk of ocular injuries in adults? Ophthalmology. 2003;110:1082-1088.
10. Zinreich SJ, Miller NR, Aguayo JB, Quinn C, Hadfield R, Rosenbaum AE. Computed tomographic three-dimensional localization and compositional evaluation of intraocular and orbital foreign bodies. Arch Ophthalmol. 1986;104:1477-1482.
11. Lindahl S. Computed tomography of intraorbital foreign bodies. Acta Radiol. 1987;28:235-240.
12. Etherington RJ, Hourihan MD. Localisation of intraocular and intraorbital foreign bodies using computed tomography. Clin Radiol. 1989;40:610-614.
13. Chacko JG, Figueroa RE, Johnson MH, Marcus DM, Brooks SE. Detection and localization of steel intraocular foreign bodies using computed tomography. A comparison of helical and conventional axial scanning. Ophthalmology. 1997;104:319-323.
14. Lakits A, Prokesch R, Scholda C, Bankier A. Orbital helical computed tomography in the diagnosis and management of eye trauma. Ophthalmology. 1999;106:2330-2335.
15. Dass AB, Ferrone PJ, Chu YR, Esposito M, Gray L. Sensitivity of spiral computed tomography scanning for detecting intraocular foreign bodies. Ophthalmology. 2001;108:2326-2328.
16. Fisher YL. Advances in contact ophthalmic ultrasonography: Ocular trauma and intraocular foreign body patients. Dev Ophthalmol. 1989;18:69-74.
17. Rubsamen PE, Cousins SW, Winward KE, Byrne SF. Diagnostic ultrasound and pars plana vitrectomy in penetrating ocular trauma. Ophthalmology. 1994;101:809-814.
18. Bryden FM, Pyott AA, Bailey M, McGhee CN. Real time ultrasound in the assessment of intraocular foreign bodies. Eye. 1990;4 (Pt 5):727-731.
19. Barash D, Goldenberg-Cohen N, Tzadok D, Lifshitz T, Yassur Y, Weinberger D. Ultrasound biomicroscopic detection of anterior ocular segment foreign body after trauma. Am J Ophthalmol. 1998;126:197-202.
20. Deramo VA, Shah GK, Baumal CR, et al. The role of ultrasound biomicroscopy in ocular trauma. Trans Am Ophthalmol Soc. 1998;96:355-365; discussion 365-357.
21. Lagouros PA, Langer BG, Peyman GA, Mafee MF, Spigos DG, Grisolano J. Magnetic resonance imaging and intraocular foreign bodies. Arch Ophthalmol. 1987;105:551-553.
22. LoBue TD, Deutsch TA, Lobick J, Turner DA. Detection and localization of nonmetallic intraocular foreign bodies by magnetic resonance imaging. Arch Ophthalmol. 1988;106:260-261.
23. Williams S, Char DH, Dillon WP, Lincoff N, Moseley M. Ferrous intraocular foreign bodies and magnetic resonance imaging. Am J Ophthalmol. 1988;105:398-401.
24. Williamson TH, Smith FW, Forrester JV. Magnetic resonance imaging of intraocular foreign bodies. Br J Ophthalmol. 1989;73:555-558.
25. Glatt HJ, Custer PL, Barrett L, Sartor K. Magnetic resonance imaging and computed tomography in a model of wooden foreign bodies in the orbit. Ophthal Plast Reconstr Surg. 1990;6:108-114.
26. Ambler JS, Meyers SM. Management of intraretinal metallic foreign bodies without retinopexy in the absence of retinal detachment. Ophthalmology. 1991;98:391-394.
27. Kelsey CA, King JN, Keck GM, Chiu MT, Wolfe DM, Orrison WW. Ocular hazard of metallic fragments during mr imaging at 0.06 t. Radiology. 1991;180:282-283.
28. MacCumber MW and Zanger MW. Open-Globe Injuries. Focal Points: Clinical Modules for Ophthalmologists, Module 7, 2001.
29. Pieramici DJ et al. The prognostic significance of a system for classifying mechanical injuries of the eye (globe) in open-globe injuries. J Trauma. 2003;54:750-754.
30. Scott IU, Gayer S, Voo I, Flynn HW, Jr., Diniz JR, Venkatraman A. Regional anesthesia with monitored anesthesia care for surgical repair of selected open globe injuries. Ophthalmic Surg Lasers Imaging. 2005;36:122-128.
31. Scott IU, McCabe CM, Flynn HW, et al. Local anesthesia with intravenous sedation for surgical repair of selected open globe injuries. Am J Ophthalmol. 2002;134:707-711.
32. Boscia F, La Tegola MG, Columbo G, Alessio G, Sborgia C. Combined topical anesthesia and sedation for open-globe injuries in selected patients. Ophthalmology. 2003;110:1555-1559.
33. Fedrat S and de Juan E. Timing Guidelines for Emergent Surgery in Management of Ocular Injuries and Emergencies. Philadelphia: Lippincott, Williams & Wilkins, 1998. pp 79-89.
34. Arroyo JG et al. A matched study of primary scleral buckle placement during repair of posterior segment open globe injujries. Br J Ophthalmol. 2003;87:57-78.
35. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—tap report. Treatment of age-related macular degeneration with photodynamic therapy (tap) study group. Arch Ophthalmol. 1999;117:1329-1345.
36. Spaide RF, Sorenson J, Maranan L. Combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 2003;110:1517-1525.
37. Gragoudas ES, Adamis AP, Cunningham ET, Jr., Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805-2816.
38. Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36:336-339.
39. Heier JS, Antoszyk AN, Pavan PR, et al. Ranibizumab for treatment of neovascular age-related macular degeneration: a phase i/ii multicenter, controlled, multidose study. Ophthalmology. 2006;113:642 e641-644.
40. Benson WE SJ, Sarin LK. Blunt trauma. Philadelphia: Lippincott; 1988.
41. Kelley J. Traumatic chorioretinopathies. Philadelphia: Elsevier Mosby; 2006.
42. Conrath J, Forzano O, Ridings B. Photodynamic therapy for subfoveal cnv complicating traumatic choroidal rupture. Eye. 2004;18:946-947
43. Harissi-Dagher M, Sebag M, Gauthier D, Marcil G, Labelle P, Arbour JD. Photodynamic therapy in young patients with choroidal neovascularization following traumatic choroidal rupture. Am J Ophthalmol. 2005;139:726-728.
44. Mennel S, Hausmann N, Meyer CH, Peter S. Photodynamic therapy and indocyanine green guided feeder vessel photocoagulation of choroidal neovascularization secondary to choroid rupture after blunt trauma. Graefes Arch Clin Exp Ophthalmol. 2005;243:68-71.
45. Yamamoto I, Rogers AH, Reichel E, Yates P, Duker JS. Intravitreal bevacizumab (avastin) as treatment for subfoveal choroidal neovascularization secondary to pathologic myopia. Br J Ophthalmol. 2006 Jul 26; [Epub ahead of print].
46. Bashshur ZF, Bazarbachi A, Schakal A, Haddad ZA, El Haibi CP, Noureddin BN. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006;142:1-9.
47. Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (avastin) for neovascular age-related macular degeneration. Retina. 2006;26:495-511.
48. Ladewig MS, Ziemssen F, Jaissle G, et al. [intravitreal bevacizumab for neovascular age-related macular degeneration]. Ophthalmologe. 2006;103:463-470.
49. Ladewig MS, Ziemssen F, Jaissle G, et al. [intravitreal bevacizumab for neovascular age-related macular degeneration.] Ophthalmologe. 2006 May; [Epub ahead of print].
50. Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363-372 e365.
51. Reichel E. Intravitreal bevacizumab for choroidal neovascularization and cystoid macular edema: a cost-effective treatment? Ophthalmic Surg Lasers Imaging. 2005;36:270-271.
52. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The central vein occlusion study group m report. Ophthalmology. 1995;102:1425-1433.
53. Argon laser photocoagulation for macular edema in branch vein occlusion. The branch vein occlusion study group. Am J Ophthalmol. 1984;98:271-282.
54. Cheng KC, Wu WC. Intravitreal triamcinolone acetonide for patients with macular edema due to branch retinal vein occlusion. Kaohsiung J Med Sci. 2006;22:321-330.
55. Chen SD, Sundaram V, Lochhead J, Patel CK. Intravitreal triamcinolone for the treatment of ischemic macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2006;141:876-883.
56. Chen SD, Lochhead J, Patel CK, Frith P. Intravitreal triamcinolone acetonide for ischaemic macular oedema caused by branch retinal vein occlusion. Br J Ophthalmol. 2004;88:154-155.
57. Prasad AG, Shah GK. Intravitreal triamcinolone for ischemic branch retinal vein occlusion with serous retinal detachment. Retina. 2006;26:234-236.
58. Karacorlu M, Ozdemir H, Karacorlu SA. Resolution of serous macular detachment after intravitreal triamcinolone acetonide treatment of patients with branch retinal vein occlusion. Retina. 2005;25:856-860.
59. Cekic O, Chang S, Tseng JJ, et al. Intravitreal triamcinolone injection for treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2005;25:851-855.
60. Krepler K, Ergun E, Sacu S, Richter-Muksch S, Wagner J, Stur M, Wedrich A. Intravitreal triamcinolone acetonide in patients with macular oedema due to branch retinal vein occlusion: a pilot study. Acta Ophthalmol Scand. 2005;83:600-604.
61. Ozdek SC, Aydin B, Gurelik G, Bahceci U, Hasanreisoglu B. Effects of intravitreal triamcinolone injection on macular edema and visual prognosis in central retinal vein occlusion. Int Ophthalmol. 2006 Jun 16; [Epub ahead of print].
62. Ramezani A, Entezari M, Moradian S, Tabatabaei H, Kadkhodaei S. Intravitreal triamcinolone for acute central retinal vein occlusion; a randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2006 Jun 1; [Epub ahead of print].
63. Gelston CD, Olson JL, Mandava N. Macular oedema in central retinal vein occlusion treated with intravitreal triamcinolone. Acta Ophthalmol Scand. 2006;84:314-318.
64. Jonas JB, Akkoyun I, Kamppeter B, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide for treatment of central retinal vein occlusion. Eur J Ophthalmol. 2005;15:751-758.
65. Karacorlu M, Ozdemir H, Karacorlu S. Intravitreal triamcinolone acetonide for the treatment of central retinal vein occlusion in young patients. Retina. 2004;24:324-327.
66. Cekic O, Chang S, Tseng JJ, et al. Intravitreal triamcinolone treatment for macular edema associated with central retinal vein occlusion and hemiretinal vein occlusion. Retina. 2005;25:846-850.
67. Tewari HK, Sony P, Chawla R, Garg SP, Venkatesh P. Prospective evaluation of intravitreal triamcinolone acetonide injection in macular edema associated with retinal vascular disorders. Eur J Ophthalmol. 2005;15:619-626.
68. Avitabile T, Longo A, Reibaldi A. Intravitreal triamcinolone compared with macular laser grid photocoagulation for the treatment of cystoid macular edema. Am J Ophthalmol. 2005;140:695-702.
69. Hayashi K, Hayashi H. Intravitreal versus retrobulbar injections of triamcinolone for macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2005;139:972-982.
70. Jonas JB. Intravitreal triamcinolone acetonide for treatment of intraocular oedematous and neovascular diseases. Acta Ophthalmol Scand. 2005;83:645-663.
71. Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26:279-284.
72. Spandau UH, Ihloff AK, Jonas JB. Intravitreal bevacizumab treatment of macular oedema due to central retinal vein occlusion. Acta Ophthalmol Scand. 2006;84:555-556.
73. Jaissle GB, Ziemssen F, Petermeier K, et al. [bevacizumab for treatment of macular edema secondary to retinal vein occlusion]. Ophthalmologe. 2006;103:471-475.
74. Jonas JB, Hayler JK, Sofker A, Panda-Jonas S. Regression of neovascular iris vessels by intravitreal injection of crystalline cortisone. J Glaucoma. 2001;10:284-287.
75. Silva Paula J, Jorge R, Alves Costa R, Rodrigues Mde L, Scott IU. Short-term results of intravitreal bevacizumab (avastin) on anterior segment neovascularization in neovascular glaucoma. Acta Ophthalmol Scand. 2006;84:556-557.
76. Kahook MY, Schuman JS, Noecker RJ. Intravitreal bevacizumab in a patient with neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2006;37:144-146.
77. Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of rubeosis iridis from a single bevacizumab (avastin) injection. Retina. 2006;26:354-356.
78. Grisanti S, Biester S, Peters S, Tatar O, Ziemssen F, Bartz-Schmidt KU. Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol. 2006;142:158-160.
79. Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (avastin) treatment. Retina. 2006;26:352-354.
80. Jager RD, Aiello LP, Patel SC, Cunningham ET, Jr. Risks of intravitreous injection: A comprehensive review. Retina. 2004;24:676-698.
81. D'Amico DJ, Patel M, Adamis AP, Cunningham ET, Jr., Guyer DR, Katz B. Pegaptanib sodium for neovascular age-related macular degeneration: two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology. 2006;113:1001 e1001-1006.
82. Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intravenous injections. Retina. 2004;24:S3-S19.
83. Taban M, Behrens A, Newcomb RL, et al. Acute endophthalmitis following cataract surgery: A systematic review of the literature. Arch Ophthalmol. 2005;123:613-620.
84. McDonnell PJ, Taban M, Sarayba M, et al. Dynamic morphology of clear corneal cataract incisions. Ophthalmology. 2003;110:2342-2348.
85. Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology. 2002;109:13-24.
86. Results of the endophthalmitis vitrectomy study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis vitrectomy study group. Arch Ophthalmol. 1995;113:1479-1496.
87. Kuhn F, Gini G. Vitrectomy for endophthalmitis. Ophthalmology. 2006;113:714.
88. Ng JQ, Morlet N, Pearman JW, et al. Management and outcomes of postoperative endophthalmitis since the endophthalmitis vitrectomy study: the endophthalmitis population study of western australia (epswa)'s fifth report. Ophthalmology. 2005;112:1199-1206.
89. Smiddy WE, Smiddy RJ, Ba'Arath B, et al. Subconjunctival antibiotics in the treatment of endophthalmitis managed without vitrectomy. Retina. 2005;25:751-758.
90. Iyer MN, Han DP, Yun HJ, et al. Subconjunctival antibiotics for acute postcataract extraction endophthalmitis—is it necessary? Am J Ophthalmol. 2004;137:1120-1121.
ThucAnh T. Ho, MD is a second year vitreoretinal fellow with Illinois Retina Associates/ Rush University Medical Center (Chicago) and trained as a resident in the Wills Eye Hospital Emergency Department in Philadelphia. Mathew W. MacCumber, MD, PhD is associate professor, associate chairman for research, and vitreoretinal fellowship director in the Department of Ophthalmology, Rush University Medical Center, and a partner with Illinois Retina Associates in Chicago. Dr. MacCumber has consulted for and/or received grant support from Genentech, Novartis, Eyetech/OSI, Allergan, Bausch & Lomb, and Optos, Inc. The authors have no financial interest in information presented in this article.