AGE-RELATED
MACULAR DEGENERATION
Understanding
the Role of Genetics and Nutritional
Supplementation in the
Development of AMD
Researchers
uncover new links in the campaign to stop vision loss.
BY
JUDITH RIDDLE, SENIOR EDITOR
Age-related macular degeneration (AMD) is the leading cause of blindness in the Western world in people over age 60. The disease affects 1.75 million patients in the United States, and seven million more remain at risk.1 These facts are well established.
What has not been fully established — or understood — but continues to evolve, is the research on the role of genetic and nutritional factors in the development and progression of AMD.
|
|
|
Figure 1. Complement factor H directly inhibits both the the classical and alternate pathways, and indirectly inhibits the lectin pathway. Lack of this inhibition can result in uncontrolled activation of the complement cascade. |
CANDIDATE GENE APPROACH
Researchers have long known that certain families are genetically predisposed to AMD. Knowing that fact has spurred their attempts to isolate a single gene or family of genes that might be responsible for initiating advanced forms of the disease, said Darius Moshfeghi, MD, at the May 2006 Retinal Physician Symposium.
Using the "candidate gene approach," researchers have tried to identify a heritable disease with early onset that had traits similar to AMD, such as drusen and retinal pigment epithelial (RPE) changes. Yet, no disease matched these characteristics, Dr. Moshfeghi said. AMD is characterized by late onset and polygenic origin, neither of which is ideal to identify candidate genes.
RARE BUT SIMILAR DISEASE
The logical next step for researchers was to focus on the inflammatory origins of AMD. In doing so, they discovered that drusen are a product of a localized inflammatory response following RPE injury,2,3 that "complement cascade activation" is implicated in drusen formation4 and drusen contain numerous inflammatory modulators, such as complement pathway components and the membrane attack complex, Dr. Moshfeghi said.
Although researchers could not identify a heritable disease with AMD characteristics, they did discover that the rare disease membranoproliferative glomerulonephritis type II (MPGN) had AMD-like ocular signs.5,6 The disease is characterized by uncontrolled activation of the alternative complement pathway. And the drusen seen in MPGN are indistinguishable from those seen in AMD. Recent research has shown that a point mutation in the complement factor H gene (HF1) led to MPGN in pigs.7 In fact, mice deficient in complement factor H developed severe MPGN.8
COMPLEMENT FACTOR H GENE
With these findings in mind, researchers began looking for a connection between HF1 and the origins of AMD. They discovered HF1 was a major inhibitor of three alternative complement pathways (classical, lectin and alternate) that have been shown to precipitate inflammatory processes and lead to drusen formation, Dr. Moshfeghi said (Figure 1).
"Four groups of researchers identified the location of the [HF1] gene on chromosome 1 and the corresponding tyrosine-histidine [single nucleotide] polymorphism (SNP)," Dr. Moshfeghi continued. "To date, 50% of AMD can be attributed to this SNP, with a 7.4 odds ratio for developing the disease.9"
Recently, researchers associated nonsynonymous SNPs located at chromosome 10q26 with AMD. "[They also] reported an odds ratio of 5 and a 57% attributable risk for the plekha1/loc387715 SNP — a gene that codes for a protein involved in focal lymphocyte activation, again highlighting the role of inflammation in AMD,10" Dr. Moshfeghi explained.
RISK VS. PROTECTIVE FACTORS
|
|
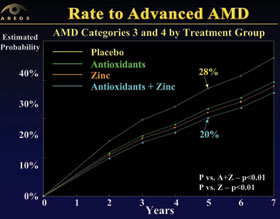
| Figure 2. Categories 3 and 4 were combined and compared across four treatment groups. The placebo group had the highest rate of progression. Both zinc alone and antioxidants plus zinc demonstrated significant improvements in the rate of progression to advanced AMD. |
Researchers also found that factor B is a major activator of the alternate complement pathway, while complement component C2 is an activator of the classical pathway.11 However, the L9H variant of complement factor B and the E318D variant of component C2 have been shown to be protective for AMD.11
"While 60% of the risk in affected patients and 65% of the protection in controls is attributable to complement factor H, 40% of the risk and 35% of the protection is attributable to C2/BF locus," Dr. Moshfeghi said. "And this is borne out by the genetic algorithm analysis demonstrating that C2 and BF contribute from 35% to 40% of the risk for AMD."
UNEXPLAINED RISK
Despite these advances in our knowledge of the role genetics play in AMD development, however, 50% of the remaining risk for the disease remains unexplained, Dr. Moshfeghi continued. As demonstrated, not all SNPs in the complement factor H gene are bad, which explains why some patients with pathogenic variants do not develop AMD. In fact, four complement factor H SNPs have been identified as protective against AMD.12
What we know for sure is that AMD risk is associated with local inflammatory insult to RPE, complement pathway activation and polygenic factors. Genes contributing risk have been identified on chromosomes 1, 6 and 10, and all are associated with either the complement pathways or lymphocyte activation.
Just as the research on genetics continues to evolve so does our knowledge of how much nutritional supplements impact AMD development.
NUTRITIONAL SUPPLEMENTS
Environmental influences, such as oxidative cell damage, have been implicated in the pathogenesis of AMD. For instance, oral zinc supplementation has been shown to decrease vision loss from the disease.13
In the Age-Related Eye Disease Study (AREDS) — the first randomized clinical trial to test the efficacy of a high-dose antioxidant-combination formula — researchers showed that patients at high risk for developing advanced AMD lowered their odds after taking vitamin C (500 mg), vitamin E (400 IU), beta-carotene (15 mg), zinc (80 mg) and copper (2 mg).14
Patients who received a combination of antioxidants plus zinc saw a 34% and 38% reduction in risk for developing advanced AMD and neovascular AMD, respectively, Dr. Moshfeghi said.
Treatment groups were divided into antioxidants, zinc, antioxidants plus zinc, and placebo. Patients with intermediate and advanced AMD in one eye realized benefits from taking antioxidants plus zinc, and zinc alone. The nutrients had no benefit, however, to those who had either no AMD or early signs of AMD, Dr. Moshfeghi continued.
ANTIOXIDANTS AND VISUAL ACUITY
Researchers also assessed the effects of antioxidants plus zinc on visual acuity (VA). The primary outcome measure included a loss of at least three lines of vision from baseline. The secondary outcomes were VA loss with an AMD event and VA worse than 20/100.
Researchers found that patients with either intermediate or advanced AMD who received the antioxidant plus zinc formulation showed a significant improvement in the primary and secondary outcomes. In fact, the antioxidant plus zinc combination was superior to placebo and other formulations for every functional outcome measure with the exception of vision worse than 20/100 (Figure 2).
CLINICAL APPLICATIONS
These results clearly suggest that physicians should prescribe antioxidants plus zinc to patients with intermediate or advanced AMD, or vision loss from AMD in one eye.
If the at-risk population in the United States took this AREDS formulation, as many as 300,000 AMD patients could avoid losing their vision.15 It's an astonishing number that has led physicians to recommend the AREDS formula to patients who fit the study criteria, Dr. Moshfeghi said.
The success of combining antioxidants and zinc to prevent vision loss has also led to questions about the role other nutrients might play. To answer these questions, Dr. Moshfeghi said, researchers will conduct an AREDS II study to evaluate the effects of dietary xanthophylls (lutein and zeaxanthin) and omega-3 fatty acids on AMD progression.
REFERENCES
1. Friedman DS, O'Colmain BJ, Muñoz B, et al. The Eye Diseases Prevalence Research Group. Arch Ophthalmol. 2004;122:564-572.
2. Hageman GS, Luthert PJ, Victor Chong NH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705-732.
3. Anderson DH, Mullins RF, Hageman GS. A role of local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411-431.
4. Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:14682-14687.
5. Mullins RF, Aptsiauri N, Hageman GS. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye 2001;15:390-395.
6. Colville D, Guymer R, Sinclair RA, et al. Visual impairment caused by retinal abnormalities in mesangiocapillary (membranoproliferative) glomerulonephritis type II ("dense deposit disease"). Am J Kidney Dis. 2003;42:E2-E5.
7. Jansen JH, Hogasen K, Harboe M. In situ complement activation in procine membranoproliferative glomerulonephritis type II. Kidney Int. 1998;53:331-349.
8. Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nature Genetics. 2002;31:424-428.
9. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385-389, 419-421, 421-424.
10. Jakobsdottir J, Conley YP, Weeks DE, et al. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005;77:389-407.
11. Nature Genetics 2006 Advance Publication.
12. Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gen factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102:7227-7232.
13. Newsome DA, Swartz M, Leone NC, et al. Oral zinc in macular degeneration. Arch Ophthalmol. 1988;106:192-198.
14. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436.
15. Bressler NM, Bressler SB, Congdon NG, et al. Potential public health impact of age-related eye disease study results: AREDS report no. 11. Arch Ophthalmol. 2003;11:1634-1636.