FUTURE
AMD THERAPIES
Attacking
Angiogenesis From All Angles
Research
continues into several promising new treatment strategies for neovascular AMD.
While the latest discoveries regarding angiogenesis in neovascular age-related macular degeneration (AMD) have already led to FDA approval of two new therapies — the most effective to date — research continues into several other treatment strategies. Updates on the latest approaches were presented at the 2006 Retinal Physician Symposium (May 31-June 3, 2006, Atlantis, Paradise Island, Bahamas). This article provides highlights from the following presentations:
► VEGF Trap (Regeneron Pharmaceuticals Inc.) and RNA interference (RNAi), Peter K. Kaiser, MD
► Triple therapy with verteporfin (Visudyne, Novartis, QLT) photodynamic therapy (PDT), bevacizumab (Avastin, Genentech) and dexamethasone, Albert J. Augustin, MD
► Potential future role of anecortave acetate (Retaane, Alcon), Jason S. Slakter, MD
► Intravitreal bevacizumab, Philip J. Rosenfeld, MD, PhD.
|
|
|
Figure 1. VEGF Trap is a "decoy" receptor fusion protein. |
VEGF TRAP
Dr. Kaiser presented an update on the investigational drug VEGF Trap for the treatment of neovascular AMD. VEGF Trap is a fusion protein that binds all forms of vascular endothelial growth factor-A (VEGF-A) and placental growth factor (PlGF). It is designed to prevent formation of new blood vessels by preventing the growth factors from interacting with cell-surface receptors.
VEGF Trap is a "decoy" receptor, meaning that it acts similar to a receptor because it can attach to VEGF, but it is made by fusing proteins onto the Fc fragment of a human domain using several domains from VEGF Receptor 1 and VEGF Receptor 2 (Figure 1). VEGF Trap binds VEGF more tightly than native receptors, monoclonal antibodies or aptamers. VEGF Trap has a systemic half-life in humans of 14 to 18 days.
"VEGF Trap is smaller than an antibody," Dr. Kaiser said. "Regeneron conducted studies similar to what was done later with bevacizumab to see if VEGF Trap penetrated the retina. The studies showed that it penetrates all the way to the choroid. This large molecule can cross the retina to treat exudative AMD. Since the exclusion limit of the retina is smaller than VEGF Trap, we still need to figure out why it can cross."
Based on the positive results of studies in animal models of choroidal neovascularization (CNV), a human study of systemically administered VEGF Trap was performed. "There appeared to be a very nice treatment effect with systemic VEGF Trap, but as the dose increased, there was also a dose-dependent increase in blood pressure, something we knew may happen with a systemic anti-VEGF agent," Dr. Kaiser explained. "This caused a dose-limiting toxicity, and development of systemic VEGF Trap for ocular disease has been halted."
|
|
| Figure 2: In the Phase 1 study of VEGF Trap, mean and median best-corrected visual acuity improved by approximately 13 letters in patients who received the two highest doses. |
However, additional preclinical animal model studies showed that treatment delivered intravitreally was effective against established CNV. The company then conducted a Phase 1 safety study with intravitreal VEGF Trap in humans. In that study, 21 patients received a single intravitreal VEGF Trap injection of either 0.05, 0.15, 0.5, 1, 2 or 4 mg. They were followed for 6 weeks, at which time they were permitted to receive other available treatments. By 6 weeks, no serious adverse events and no ocular inflammation or endophthalmitis occurred.
As measured by optical coherence tomography (OCT) posterior pole scans, the median excess retinal thickness was 194 μm at baseline and 60 μm at 6 weeks. "When you look in particular at the two highest dose groups, 2 mg and 4 mg, you see a very nice treatment effect," Dr. Kaiser said. "It does not appear to trail off at day 43." Mean preoperative best-corrected visual acuity (BCVA) in the study was 20/160. BCVA for all patients in the study increased by a mean of 4.8 letters. However, Dr. Kaiser pointed out, in the two highest dose groups the mean and median improvement in BCVA was approximately 13 letters, with three of six patients gaining 15 or more letters (Figure 2).
"VEGF Trap delivers a very nice anatomic and visual result, but why are we discussing a molecule that, for all intents and purposes, appears to be like bevacizumab," Dr. Kaiser asked. The answer, he said, lies in the fact that VEGF Trap has been shown to be present in the rabbit eye at a concentration of nearly 100 nM 1 month after intravitreal injection of 500 μg.
In addition, it effectively binds VEGF at low ambient concentrations. "By combining the levels we get at 1 month and longer with its very high ability to bind VEGF, maybe we can get a much longer duration of action with VEGF Trap than we are seeing with other drugs," he said.
|
|
|
Figure 3. Safety of a single injection of Sirna-027 in the Phase I study. |
Based on the Phase 1 study results, Regeneron has initiated a 150-patient, 12-week, Phase 2 trial of VEGF Trap in neovascular AMD. The trial is designed to evaluate safety and efficacy as well as repeat injections using different doses and dosing regimens.
RNAI
Dr. Kaiser also presented an update on two antiangiogenesis therapies that employ the strategy of RNAi. Unlike the protein antagonist therapies, such as ranibizumab, which act on VEGF after it has been produced, RNAi therapies prevent VEGF or VEGF receptor production, he said.
When double-stranded RNA is injected into a cell, it attaches to and activates a cellular protein complex, the RNA-induced silencing complex (RISC). The activated RISC protein searches for complementary messenger RNA (mRNA) and breaks it down. "But it does not just do it once," Dr. Kaiser explained. "It goes out and finds all the other mRNAs in the cell and continues to break them down until the RISC protein itself breaks down. That is one nice aspect of this technology. The other is its specificity. You can target specific genes."
Previously, three separate efforts were under way to develop RNAi therapies for neovascular AMD. One of those efforts, a partnership between Merck and Alnylam Pharmaceuticals has been dropped, leaving Acuity Pharmaceuticals and Sirna Therapeutics/Allergan still working in this arena. According to Dr. Kaiser, the Acuity compound acts by preventing VEGF production, while the Sirna/Allergan compound acts by preventing production of a VEGF receptor.
SIRNA-027
|
|
| Figure 4. Visual acuity results from the Phase I study of bevasiranib siRNA therapy. Patients received an intravitreal injection at day 0 and week 6. |
Sirna Therapeutics' Sirna-027 was tested in a Phase 1, open-label, dose-escalation (100 μg to1600 μg) study. At 8 weeks after a single intravitreal injection, all 26 patients (100%) showed visual acuity stabilization, and five patients (19%) experienced clinically significant improvement in visual acuity (three lines or more). In 4% of patients, vision deteriorated. At 12 weeks after a single injection, 24 of 26 patients (92%) showed visual acuity stabilization, and four patients (15%) experienced clinically significant improvement in visual acuity. A reduction in visual acuity of three lines or more occurred in two (8%) patients. No serious adverse events or dose-limiting toxicities occurred (Figure 3).
Based on the Phase 1 results, a Phase 2 study in partnership with Allergan called SIRIUS is planned to evaluate multidose treatment.
Bevasiranib
Acuity Pharmaceuticals' RNAi therapy, bevasiranib (formerly known as Cand5), was evaluated in a Phase 1, open-label, pharmacokinetics, dose-escalation study involving 15 patients. The study, the first use of small interfering RNA (siRNA) in humans, was designed to establish the safety profile, tolerability and preliminary efficacy of five escalating doses of bevasiranib delivered by intravitreal injection.
Participation criteria included visual acuity of 20/50 to 20/320, subfoveal CNV due to AMD and predominantly classic, minimally classic or occult lesions 12 disc areas. Patients received an injection at day 0 and week 6. The maximum dose tested was 3.0 mg.
|
|
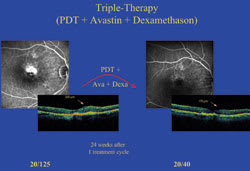
Figure 5. In this patient, 24 weeks after 1 cycle of triple therapy for a minimally classic lesion, retinal thickness improved from 300 μm to 170 μm and visual acuity improved from 20/125 to 20/40. |
"The safety in this study was excellent," Dr. Kaiser said. No patients were withdrawn due to adverse events. The most commonly reported ocular adverse events were injection-related, such as subconjunctival hemorrhage, ocular pain and vitreous floaters, and described as mild. Blood samples were obtained from all patients at 30 minutes and 24 hours following injection. The plasma samples obtained were assayed for levels of bevasiranib, which was not detected in any of the samples at all five dose groups.
At week 6, 100% of patients had stable or improved vision, and no patients lost three or more lines of vision (Figure 4). "This effect was persisting out to week 12," Dr. Kaiser said.
A Phase 2, randomized, double-masked study, called CARE, has completed enrollment and is nearing completion. It is comparing three doses of bevasiranib in 120 patients over a period of 12 weeks.
"So we do have other ways to block VEGF in addition to Avastin, Lucentis and Macugen," Dr. Kaiser said. "The reason these other products are continuing to be tested is that they may last longer in the eye and increase our dosing interval without decreasing efficacy."
TRIPLE THERAPY FOR CNV IN AMD
|
|
| Figure 6. In this patient, 24 weeks after one cycle of triple therapy for a juxtafoveal lesion that was leaking into the fovea, retinal thickness improved from 555 μm to 330 μm and visual acuity improved from 20/80 to 20/40. |
Dr. Augustin presented the results of a single-center case series testing the hypothesis that a combination of three therapies — verteporfin photodynamic therapy (PDT), bevacizumab and dexamethasone — is safer and more finite than other treatments for AMD-related CNV.
Explaining the hypothesis, Dr. Augustin said, "VEGF expression is at least a byproduct of inflammation. We all know that anti-VEGF monotherapy is more a treatment of CNV side effects until the CNV becomes quiescent, and we all know that PDT monotherapy has no anti-inflammatory effect and no anti-VEGF effect. So the side effects of the CNV are not antagonized when we use PDT as a CNV terminator."
The goals of triple therapy, he said, are to extinguish the effects of CNV and restore visual acuity with one treatment cycle rather than multiple retreatments, and to avoid creating a chronic disease state.
Mean baseline visual acuity among the 59 patients in the study was 20/125. Mean lesion size at baseline was 2600 μm. All lesion compositions were included from 0% to 100% classic (34.7% predominantly classic, 26.5% minimally classic, 38.8% occult). Approximately 70% of the lesions were subfoveal; 29% were juxtafoveal; and 2% were extrafoveal. All patients received modified PDT (light dose 42 J/cm2 accomplished by light delivery time of 70 seconds to prevent choroidal infarction) and 1.25 mg of intravitreal bevacizumab followed 18 hours later by 800 μg of intravitreal dexamethasone. Mean follow-up time was 28 weeks.
After only one treatment cycle, a mean increase in visual acuity of 1.9 lines occurred (p<0.01) as did a mean decrease in retinal thickness of 183.7 μm (p<0.01). No steroid-related or other side effects occurred. Leakage did not recur in any patient, but nine patients received an additional injection of bevacizumab for macular edema. All nine of those patients responded well to the additional injection and none had received any further treatment at the time of Dr. Augustin's presentation in June 2006.
"This triple therapy leads to a significant visual acuity
increase using only one treatment cycle," Dr. Augustin said. (Figures 5, 6, 7) "We
believe that this
is a more finite treatment. It is more of a cure than a suppression
of the disease process."
Dr. Augustin also said that further evaluation is needed to determine ideal drug doses, PDT light dose, and timing between the different treatments. In addition, future studies of the triple therapy could use FDA-approved, rather than off-label, drugs.
ANECORTAVE ACETATE
Dr. Slakter addressed the future potential role of anecortave acetate in the AMD treatment armamentarium.
Reviewing anecortave's unique characteristics, Dr. Slaker explained that it is in a new class of compounds, called cortisenes, engineered to control angiogenesis. Being a synthetic analogue of cortisol, anecortave is devoid of glucocorticoid receptor-mediated activity and does not elevate intraocular pressure (IOP) or cause cataracts. "The important thing to keep in mind about anecortave, especially if we talk about its potential future role, is where it acts in the angiogenic cascade," Dr. Slakter said (Figure 8).
"Anecortave acts differently than other antiangiogenesis molecules. It reduces the mRNA translation and production of molecules such as VEGF and insulin-like growth factor-1," he continued. "In addition, and very importantly, it acts downstream, within the interior of the cell, independently of initiating stimuli, preventing generation of matrix metalloproteinases, which are critical for the breakdown of the extracellular matrix and resultant neovascularization."
Anecortave acetate has been studied in more than 10 preclinical models, showing significant inhibition of neovascularization in all of them. "In most of these studies, anecortave was applied as a risk-reducing or preventive agent rather than an after-the-fact agent following induction of CNV," Dr. Slakter said.
He also reviewed the results of C-01-99, the large clinical trial that compared anecortave acetate head-to-head with verteporfin PDT. While that study failed to meet its endpoint of noninferiority to PDT, it did demonstrate a durable treatment effect and a good safety profile. "Anecortave generated a rather flat curve in visual acuity over the course of the clinical trial, suggesting that it can maintain long-term stability of neovascularization both anatomically and visually," Dr. Slakter said. "Safety-wise, there were no significant systemic or local adverse events related to the drug. Anecortave acetate is also well-suited for long-term therapy because it is dosed less frequently than other therapies, every 6 months, and is safely administered by posterior juxta-scleral depot outside the eye."
|
|
|
Figure 7. In this patient, 15 weeks after one cycle of triple therapy for a small lesion, CNV completely regressed, retinal thickness improved from 450 μm to 125 μm, and visual acuity improved from 20/40 to 20/25. |
Furthermore, because of its multiple mechanisms of action, anecortave acetate may be useful as part of a combination therapy, Dr. Slakter said. In the C-00-07 clinical trial, during which patients received PDT treatment and one injection of anecortave acetate, anecortave reduced the need for PDT retreatment at 6 months, and patients who received both treatments showed a trend toward better visual outcome than patients receiving multiple PDT treatments alone. "C-00-07 was a small study to which we cannot attach much meaning; nevertheless, it showed that PDT and anecortave could be safely combined and it gives us something interesting to explore in the future," he said.
The life cycle of CNV opens other possibilities for future anecortave use, Dr. Slakter said. "Early in the development of neovascularization, VEGF is an important mediator. However, as time passes and the vessels mature, things change. Other growth factors become important in the maintenance of the neovascular process and may affect the way the different antigrowth agents act and may help to explain the variability of effect throughout the duration of disease." Therefore, he continued, "the more places you can strike at the disease cascade, the better the chances of reducing progression of the disease."
A new trial involving anecortave acetate as part of combination therapy is planned. The BRIDGE study, a collaboration among the National Eye Institute, Alcon and Genentech, will evaluate the ability of anecortave to reduce the number of retreatments with ranibizumab while improving or maintaining visual acuity.
Retinal specialists are also awaiting results from the Anecortave Acetate Risk Reduction Trial (AART), for which enrollment is complete. "The risk reduction trial to me is the most important because we need to get this disease under control before it starts," he said. Key AART endpoints are the percentage of anecortave-treated patients developing sight-threatening CNV in the study eye and the percentage of anecortave-treated patients with a three-line decrease in visual acuity due to sight-threatening CNV.
Dr. Slakter summarized his presentation by saying that because of its delivery approach, safety profile and mechanism of action, anecortave acetate is uniquely positioned to be used as a maintenance therapy, in combination with other treatments, and to reduce the development of neovascularization in patients at high risk.
BEVACIZUMAB
In his update on bevacizumab for the treatment of neovascular AMD, Dr. Rosenfeld said the off-label use of the drug is legal and ethical as long as physicians use solid clinical judgment and thoroughly discuss the risks with patients. "Obviously we need prospective clinical trials," he said. "But the treatment appears to be safe and effective."
Dr. Rosenfeld reviewed the history of bevacizumab use in ophthalmology. Shortly after FDA approval of the drug for metastatic colorectal cancer in February 2004, Dr. Rosenfeld and his colleagues at Bascom Palmer Eye Institute in Miami were the first to evaluate its off-label use in AMD patients.
In the open-label Systemic Avastin for Neovascu-lar AMD (SANA) trial, intravenous treatment resulted in significant and rapid improvements in visual acuity and retinal thickness as measured by OCT. No serious adverse events occurred among the 18 SANA patients, although approximately half of them required medication or a medication change for systemic hypertension. Also in 2004, the FDA issued a letter to physicians warning them that bevacizumab use in cancer patients doubled the risk of serious arterial thromboembolic events.
Ongoing concern about the risks of using bevacizumab systemically led Dr. Rosenfeld and others to explore the possibility of reducing the drug dosage for neovascular AMD patients. Dr. Rosenfeld discovered that a smaller amount of bevacizumab, 400 times less than the dose used in cancer patients, would be nearly equivalent to the dose of ranibizumab that at the time was being tested intraocularly in clinical trials. Dr. Rosenfeld hypothesized that this amount could be safely injected directly into the eye. Lower dosage also meant lower cost, a major benefit for patients.
In May 2005, Dr. Rosenfeld treated the first AMD patient with intravitreal bevacizumab. The case report was published in July.1 Within 6 months, retinal specialists around the globe were using the drug in this manner.
At the 2006 meeting of the Association for Research in Vision and Ophthalmology, more than 80 papers detailing research and clinical experience with intravitreal bevacizumab were presented. Those and subsequent papers include toxicity studies in animals and in vitro as well as safety and efficacy studies in animals and humans.
"Overall, in the retrospective analyses that have been done, which have follow-up of 2 to 3 months, initial visual acuity is approximately 20/200 and final visual acuity is approximately 20/100," Dr. Rosenfeld said. "Also, there is an overall decrease in retinal thickness of about 100 microns, and no serious ocular or systemic adverse events have been identified." In the Bascom Palmer retrospective review (n=53), Dr. Rosenfeld said, "We were able to identify three-line gainers to 3 months, and 44% of patients gained three lines. Within 1 week, vision improved by approximately six letters; by 3 months it plateaus at eight letters. Twenty percent of patients received only one injection."
Dr. Rosenfeld also explained The International Intravitreal Bevacizumab (Avastin) Survey, an Internet-based survey that was designed to gauge the incidence of adverse events related to use of intravitreal bevacizumab for neovascular AMD and other ocular diseases.2 The survey was sent to retinal specialists worldwide who are using bevacizumab.
|
|
|
Figure 8. Anecortave acetate works upstream of growth factor-induced receptor stimulation. It also acts by regulating gene activities of vascular endothelial cells that affect various downstream processes such as the production of matrix metalloproteinases. |
Responses to the survey came from 70 centers in 12 countries that collectively performed 7113 injections in 5228 patients. Only one case of endophthalmitis and three cases of retinal detachment were reported. "There was some low-grade inflammation detected, but nothing of significance," Dr. Rosenfeld said. "The thromboembolic events do not appear to be significant compared to the rates one would expect in a population this size." Dr. Rosenfeld pointed to the limitations of the survey, including that it involved self-reporting, was voluntary, and represents short-term follow-up of approximately 3 months. Nevertheless, he said, "no apparent safety signals were identified."
While Medicare has no national policy governing the off-label use of bevacizumab in AMD, the majority of states have policies that allow Medicare coverage based on appropriate documentation. "We were able to convince our carrier in Florida to accept OCT rather than fluorescein angiographic documentation," Dr. Rosenfeld said. "That is good news for patients because we had been performing angiograms just to have documentation for Medicare."
In closing, Dr. Rosenfeld advised physicians who are using bevacizumab off-label to tailor the treatment to the individual patient with neovascular AMD. Each patient is different and the need for retreatment is unpredictable. "You cannot look at a patient on day 1 and tell whether that patient is going to be a fast responder with great durability or a patient who needs more frequent injections," he said.
REFERENCES
1. Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331-335.
2. Fung AE, Rosenfeld PJ, Reichel E. The international intravitreal bevacizumab safety survey: using the Internet to assess drug safety worldwide. Br J Ophthalmol. 2006;published online 19 Jul. doi:10.1136/bjo.2006.099598.














