PEER
REVIEWED
Evolutionary and Revolutionary Trends in Vitreoretinal Surgery
PAWAN
BHATNAGAR, MD, HOWARD F. FINE, MD, MHSc, & I-VAN HO, MBBS
|
|
|
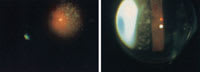
Figure 1. Intraoperative photographs of technique to stain theinternal limiting membrane while protecting the macular hole with a bubble of perfluorocarbon liquid. This prevents the ICG from reaching the RPE or subretinal space during staining. |
INTRODUCTION
In the 35 years that have elapsed since Machemer first reported successful closed vitreoretinal surgery, the field has seen tremendous growth.1 The advent of vitreoretinal surgery has allowed for the management of previously incurable conditions and has expanded the treatment options in others. Herein, we describe the revolutionary and evolutionary trends in vitreoretinal surgery that have and will continue to define the face of the field in the coming years. Revolutions are defined as advances that are major steps forward that brought or will bring widespread change in surgical practice, while evolutions are those that reflect the continued refinement of surgical patient care. As innovations in technology and equipment often pave the way for progress in surgical techniques, we will begin our discussion with the changes in surgical equipment that have and will continue to redefine vitreoretinal surgery.
HEAVY LIQUIDS
The introduction of perfluorocarbon liquids into the surgical arena has spurred a new era in retinal surgery.2 Beyond having revolutionized the treatment of retinal detachments with giant tears by obviating the need for the surgeon to operate in the supine position, it has also affected great change in the management of many other conditions. New evolutionary applications of this compound have been devised to harness perfluorocarbon liquid as a "third instrument." These approaches include its use in aiding the management of dislocated lenses, in the removal of intraocular foreign bodies, and for stabilization of bullous retinal detachments during vitreous base shaving. In subretinal or suprachoroidal hemorrhages, perfluorocarbon liquids are used to displace hemorrhage through retinotomies or sclerotomies for evacuation.3,4 Others have used perfluorocarbons in macular hole surgery for safer application of indocyanine green (ICG) to stain the inner limiting membrane (ILM) while minimizing the risk of retinal pigment epithelium (RPE) exposure with good results (Figure 1). Its use as a short-term tamponade of inferior retinal pathologies is also an area of continued investigation.5
A natural evolutionary focus includes the search for perfluorocarbon-related liquids with safer profiles for longer-term postoperative tamponade of inferior retinal breaks.5 Though the search for an ideal compound continues, some progress has been made. The recent introduction of heavy oil mixtures as inferior tamponade agents has shown initial promise in the management of cases with proliferative vitreoretinopathy as well as those with inferior retinal breaks.6-8 Although attractive as a tamponade agent, the long-term effects of heavy silicone oil are unknown and its subsequent removal from the eye is a very challenging maneuver, particularly in the setting of an underlying re-detached retina.
|
|
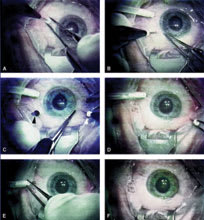
| Figure 2. This patient presented with an idiopathic epiretinal membrane and preoperative visual acuity of 20/100. A. Placement of the first microcannula. The microcannula is being held by its collar to stabilize it while the trocar is withdrawn. B. An infusion cannula has been placed in the infe-rotemporal quadrant. No suture is required to hold it in place, because it fits tightly into the microcannula. In the same frame, a second microcannula is being placed. The insertion of the microcannula is transconjunctival, and no previous dissection is required. C. After insertion of the second microcannula, its orifice was temporarily closed with a plug (black arrow), and a third microcannula was inserted (white arrow). D. After vitrectomy and membrane peel, all the microcannulae were simply removed. No suture was required at any conjunctival and scleral opening site. In this frame, the superonasal microcannula is being removed.E. Last, the inferotemporal microcannula is being removed in conjunction with the infusion cannula held by its collar.F. The eye immediately after the removal of all microcannulae. This sutureless and self-sealing system allowed for minimal postoperative discomfort and hastened the recovery by minimizing the surgically induced trauma. Intraocular pressure at first postoperative visit was 12 mm Hg. Visual acuity was measured at 1 week in this case and improved to 20/40. |
SUTURELESS SURGERY
A recent revolutionary trend in vitreoretinal surgery was the introduction of the transconjunctival sutureless25-gauge vitrectomy system (Figure 2).9 This technique has allowed for reduced surgical trauma, duration of surgery, and postoperative healing time. The success of 25-g vitrectomy has been well described, but its application for more complex vitreoretinal diseases, such as complex retinal detachment with proliferative vitreoretinopathy, has been limited. The main limiting factors with the 25-gsystem are the relative lack of instrument rigidity, slower vitreous cutting ability, and suboptimal fluidics inherent to the reduced caliber of the instrumentation. The risk of postoperative hypotony from leaking wounds that persist despite partial fluid-air exchange has been reported as well.
Some retinal surgeons have described the technique of 20-g sutureless vitrectomy to overcome some of the limitations of 25-g systems, but inconsistencies in the application of this technique have limited its widespread use. This has led to the evolutionary compromise between these two techniques: 23-g transconjunctival sutureless vitrectomy.10 This system combines the advantages of decreased surgical trauma and recovery time enjoyed with 25-g sutureless vitrectomy with the sturdier instrumentation and improved fluidics of the 20-g vitrectomy systems. These characteristics make 23-g vitrectomy a promising approach to efficiently and safely tackle the complete range of vitreoretinal surgical procedures with a single system (Figure 3).
VISUALIZATION
Optimal visualization is the starting point of any successful retinal surgery; as such, the development of wide-angle viewing systems has greatly expanded the scope and safety of vitreoretinal surgery. Today, the 2 types of wide-angle systems include both contact and noncontact lenses.11 Both deliver a large field (up to 150Þ) of view during vitreoretinal surgery, leading to a safer and more complete vitrectomy. Modern contact lenses include those that are more compact, allowing the surgeon to visualize the scleral ports at all times, hence facilitating the insertion and removal of instruments without removal of the lens (Figure 4).12 Newer lenses also ease indented examination of the peripheral retina, providing better access to peripheral fundus pathologies. Increasingly complex vitreoretinal maneuvers are also made possible by the smaller diameter contact lenses, allowing for further instrument mobility. These developments combine to improve the efficiency of vitrectomy surgery.
ENDOILLUMINATION
The evolution of endoillumination systems was driven by the need for a brighter, wider, and more even field of illumination to utilize the wider fields newer lenses have provided. Evolution to modern xenon arc lighting has brought vivid and uniform illumination, while reducing the reflections and scattering glare encountered in air-filled eyes. This is particularly important in the current trend toward smaller-gauge (23- and 25-g) sutureless vitrectomy techniques, which have inherently lowered illumination compared with 20-g systems. Other advances have included the addition of bullet probes to enhance wide-field endo-illumination, shielded bullet probes to provide up to 180Þ of illumination while controlling glare (Figure 5),13 and more recently, chandelier lighting systems (Figure 6).14 Lighted instruments likewise have been introduced to the surgical armamentarium to further ease complex tissue manipulations by aiding bimanual surgery. Potential improvements on current systems include the use of slit-lamp illumination combined with a wide-angle contact lens during vitrectomy to provide a wide field of view and sufficient illumination. This approach obviates the need for light pipe illumination, thereby enhancing the use of bimanual surgical techniques (Figure 7).15
NOVEL SURGICAL DEVICES
The ability to address more complex retinal pathologies as outlined above, including those with retinal neovascularization and preretinal fibrous proliferation, has also brought greater attention to the intraoperative difficulties some of these cases pose. In addition to bleeding, the dissection of epiretinal tissue is often fraught with the creation of iatrogenic retinal breaks. Investigations have aimed to equip surgeons with intraocular tools with multiple functions to better meet these challenges and ease bimanual surgery. Ideally they would also minimize the need for frequent introduction and removal of instruments from the eye. Progress is reflected in the creation of lighted vitrectomy instruments and multifunction tissue manipulators with diathermy and aspiration.
|
|
|
Figure 3. Technique for tunnel incision and insertion of the microcannulae. A. 23-g stiletto blade is inserted transconjunctivally into the sclera at a 30° to 45° angle. B. The inserter is introduced into the tunnel incision. C. To insert the microcannulae into the tunnel, the inserter is moved from its original tangential position into a position perpendicular to the scleral surface, where it exerts the necessary pressure on the globe. D. Microcannula in place within the tunnel incision after removal of the inserter. |
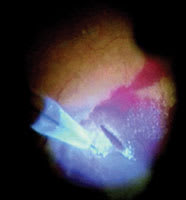
Recognizing that retinal traction created by epiretinal tissue dissections predispose to bleeding and breaks, the pulsed electron avalanche knife (PEAK-fc) was designed to eliminate these surgically created forces. This novel electrosurgical knife has, in initial reports, been capable of precisely dissecting tissue without the use of traction. By harnessing pulsed plasma-mediated discharges, it has allowed for accurate and controlled retinal incisions and tissue dissections. Furthermore, its design has incorporated both illumination and coagulation capabilities to optimize its functionality as a surgical tool (Figure 8).16 Continued experience with, and greater availability of, this tool will help identify its safety profile for intraocular use and its range of application. (Currently it is not available commercially.)
NEW SURGICAL TECHNIQUES
As the tools needed to perform vitreoretinal surgery have become increasingly more ergonomic, efficient, and reliable, the spectrum of surgical indications and techniques has expanded. Perhaps the best and most successful example of this principle has been the description of vitrectomy and subsequent gas tamponade for the closure of macular holes.17 This revolutionary procedure has allowed for the successful visual rehabilitation of countless patients who previously had incurable conditions.18 While the initial report achieved a then-astounding 58% closure rate, successful application of vitrectomy for macular holes has seen an increase to greater than 90% success. This increased closure rate reflects the continuing evolution of the surgical technique. Though not endorsed by all surgeons, ILM peeling is a radical modification introduced to the management of macular holes. The choice of tamponade agent has also varied by authors. The continued improvement of macular hole surgery includes shorter positioning times after surgery to ease patient recovery; some authors advocate only 4 days of face-down positioning while others have attempted, with some success, no positioning at all.19,20 Increased experience will lead to the further evolution of this already successful surgery with increasing closure rates and decreasing patient recovery times.
Improved vision after successful macular hole closure reflects the relative preservation of the retinal photoreceptors and the underlying RPE. It is, however, the lack of a healthy underlying RPE in age-related macular degeneration (AMD) that led to the pioneering surgical technique of macular translocation.21 This revolutionary concept, though used less commonly today due to improved pharmacologic treatments for choroidal neovascularization, represented a major paradigm shift in vitreoretinal surgery. Another revolutionary technique proposed for the management of AMD was that of submacular surgery. At the time of its initial description in 1988, there were essentially no effective treatments for submacular hemorrhage.22 The novel concept of intentionally creating posterior macular incisions to gain access to the submacular space mandated innovation in technique and instrumentation. Though revolutionary at the time of their initial descriptions, the importance of macular translocation and submacular surgery as techniques may still have even greater import in future experimental treatments, such as stem-cell transplantation or in the placement of artificial visual implants.
|
|
| Figure 4. New wide-angle viewing lens, ClariVIT (left) and the section of ClariVIT (right). ClariVIT is partially truncated, and the diameter is greatly reduced. This section of ClariVIT shows the relationship of two bonded parts: PMMA and glass. |
Current trends in vitreoretinal surgery, as in other surgical specialties, revolve around the minimization of surgical trauma. This minimally invasive shift in vitreoretinal practice has led to decreased patient recovery times and less postoperative discomfort. Current efforts to further diminish iatrogenic injury include the experimental use of enzymes or chemically active substances to create and/or facilitate vitrectomy. The use of plasmin prior to vitrectomy has been shown to create a posterior vitreous detachment in a substantial percentage of cases.23,24 These agents are being evaluated as adjuncts to vitrectomy to ease the vitreoretinal traction induced during intraoperative posterior hyaloid separation. Even less invasive is the concept of pars plana injection of these agents as monotherapy for conditions such as vitreous hemorrhage.25
PHARMACOTHERAPY-ASSISTED SURGERY
The importance of pars plana delivered anti-vascular endothelial growth factor (VEGF) therapy in retinal and choroidal neovascular processes, as well as in certain venous occlusive states,26 has recently gained attention for non-surgical conditions. The ability of these agents to induce regression of new vessels has been increasingly documented in the literature.27 Theoretically it is an attractive concept to inject anti-VEGF agents prior to surgery in an effort to minimize intraoperative bleeding in eyes with retinal proliferative diseases. Future studies will hopefully determine the safety and efficacy of these medications for this indication.
|
|
|
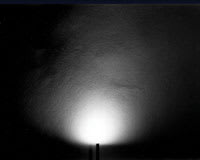
Figure 5. The wide-angle endoilluminator. Note almost 180° spread of the light from the tip of the probe. |
The revolutionary off-label use of intravitreal triamcinolone acetonide (IVTA) injection in many ways has paved the way for our clinical use of pharmacologic agents. The evolution of IVTA use has affected surgical practice as well. As an adjunct during surgery, IVTA has proved to be an effective marker to highlight residual vitreous on the retinal surface. In the same manner, substances such as ICG and trypan blue, though initially meant for other ocular uses, have become integral tools to successful vitreoretinal surgery in off-label capacities.
CONCLUSION
In summary, vitreoretinal surgery is a specialty that has rapidly progressed and that continues to incorporate new technologies and techniques. Revolutionary changes have allowed for the successful management of previously inoperable states, and they have served as the impetus for continued evolution. The spectrum of vitreoretinal surgical indications has grown because of a wider array of instrumentation with improved safety profiles. These improved abilities have allowed for the attempted repair of more severe conditions that have necessitated, out of their complexity, further surgical innovation to optimize patient outcomes. The future may bring robotic-assisted vitreoretinal surgery for fine manipulations not currently achievable by humans; artificial vision with retinal, choroidal, or cortical implants; advances in pharmacotherapy-assisted surgical techniques, including inhibitors of proliferative vitreoretinopathy and gene therapy; and other techniques that we have yet to imagine. It is clear that current trends will continue to redefine vitreoretinal surgical procedures and that an exciting era of minimally invasive, yet maximally effective, surgical approaches is on the horizon.
|
|
| Figure 6. Schematic of Awh Chandelier, providing wide-field illumination to allow for bimanual surgery with a low risk of macular phototoxicity due to the significant distance between the light source and the posterior pole. |
REFERENCES
1. Machemer R, Parel JM, Norton EW. Vitrectomy: a pars plana approach. Technical improvements and further results. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:462-466.
2. Chang S. Perfluorocarbon liquids in vitreoretinal surgery. Int Ophthalmol Clin. 1992;32:153-163.
3. Kamei M, Tano Y, Maeno T, Ikuno Y, Mitsuda H, Yuasa T. Surgical removal of submacular hemorrhage using tissue plasminogen activator and perfluorocarbon liquid. Am J Ophthalmol. 1996;121:267-275
4. Soheilian M, Peyman GA, Wafapoor H, Navarro GC, Thompson H. Surgical management of traumatic retinal detachment with perfluorocarbon liquid. The Vitreon Study Group. Int Ophthalmol. 1996-1997;20:241-249.
5. Kobuch K, Menz IH, Hoerauf H, Dresp JH, Gabel VP. New substances for intraocular tamponades: perfluorocarbon liquids, hydrofluorocarbon liquids and hydrofluorocarbon-oligomers in vitreoretinal surgery. Graefes Arch Clin Exp Ophthalmol. 2001;239:635-642
6. Tognetto D, Minutola D, Sanguinetti G, Ravalico G. Anatomical and functional outcomes after heavy silicone oil tamponade in vitreoretinal surgery for complicated retinal detachment: a pilot study. Ophthalmology. 2005;112:1574.
|
|
| Figure 7. (Left) Fundus view illuminated by a light pipe through a wide angle viewing contact lens. (Right) Fundus viewing illuminated by a slit lamp through a wide angle viewing contact lens. |
7. Rizzo S, Genovesi-Ebert F, Belting C, et al. Long-term vitreous replacement with perfluorohexyloctane and silicone oil: preliminary reports of a multicentric study. Ophthalmologica. 2005;219:147-153.
8. Wolf S, Schon V, Meier P, Wiedemann P. Silicone oil-RMN3 mixture ("heavy silicone oil") as internal tamponade for complicated retinal detachment. Retina. 2003;23:335-342.
9. Fujii GY, De Juan E Jr, Humayun MS, et al. Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery. Ophthalmology. 2002;109:1814-1820.
10. Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina. 2005;25:208-211.
11. Bovey EH, Gonvers M. A new device for noncontact wide-angle viewing of the fundus during vitrectomy. Arch Ophthalmol. 1995;113:1572-1573.
12. Nakata K, Ohji M, Ikuno Y, et al. Wide-angle viewing lens for vitrectomy. Am J Ophthalmol. 2004;137:760-762.
13. Peyman GA, Canakis C, Livir-Rallatos C, Easley J. A new wide-angle endoillumination probe for use during vitrectomy. Retina. 2002;22:242.
14. Chow DR. Shedding some light on current endoillumination. Retinal Physician. 2005;2:37-39.
15. Ohji M, Tano Y. Vitreoretinal surgery with slit-lamp illumination combined with a wide-angle-viewing contact lens. Am J Ophthalmol. 2004;137:955-956.
16. Priglinger SG, Haritoglou C, Mueller A, et al. Pulsed electron avalanche knife in vitreoretinal surgery. Retina. 2005;25:889-896.
|
|
| Figure 8. Drainage retinotomy on attached retina. Patient had minor subretinal bleeding with age-related macular degeneration. Extrafoveolar retinotomy on attached retina was performed, and subretinal bleeding and choroidalneovascularizations were removed successfully. Pulsedelectron avalanche knife parameters (determined for safe dissection of the neurosensory retina 15): voltage, 350 V; pulse repetition rate, 30 Hz; pulse duration, 100 microseconds; and 30 minipulses per pulse. |
17. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109:654-659.
18. Williams GA. Macular holes: the latest in current management. Retina. 2006;26(6 Suppl):S9-S12.
19. Park DW, Sipperley JO, Sneed SR, Dugel PU, Jacobsen J. Macular hole surgery with internal-limiting membrane peeling and intravitreous air. Ophthalmology. 1999;106:1392-1397.
20. Tornambe PE, Poliner LS, Cohen RG. Definition of macular hole surgery end points: elevated/open, flat/open, flat/closed. Retina. 1998;18:286-287.
21. Machemer R, Steinhorst UH. Retinal separation, retinotomy, and macular relocation: II. A surgical approach for age-related macular degeneration? Graefes Arch Clin Exp Ophthalmol. 1993;231:635-641.
22. de Juan E Jr, Machemer R. Vitreous surgery for hemorrhagic and fibrous complications of age-related macular degeneration. Am J Ophthalmol. 1988;105:25-29.
23. Rizzo S, Pellegrini G, Benocci F, Belting C, Baicchi U, Vispi M. Autologous plasmin for pharmacologic vitreolysis prepared 1 hour before surgery. Retina. 2006;26:792-796.
24. Williams JG, Trese MT, Williams GA, Hartzer MK. Autologous plasmin enzyme in the surgical management of diabetic retinopathy. Ophthalmology. 2001;108:1902-1905.
25. Hermel M, Mahgoub M, Youssef T, et al. Safety profile of the intravitreal streptokinase-plasmin complex as an adjunct to vitrectomy in the rabbit. Graefes Arch Clin Exp Ophthalmol. 2006;244:996-1002.
26. Iturralde D, Spaide RF, Meyerle CB,et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26:279-284.
27. Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275-278.
Pawan Bhatnagar, MD, Howard F. Fine, MD, MHSc, and I-Van Ho, MBBS, practice ophthalmology at Vitreous Retina Macula Consultants of New York. Dr. Fine is also a postdoctoral clinical fellow with the Harkness Eye Institute of the Columbia University College of Physicians and Surgeons in New York. None of the authors has any financial interest in any of the information contained in this article. They can be contacted at (212) 861-9797.