PEER REVIEWED
Advances
in Visualization During Vitrectomy Surgery
KRISTIE
L. LIN, MD, & HOWARD F. FINE, MD, MHSc
INTRODUCTION
Visualization is crucial in surgery, and ophthalmology is no exception. However, vitrectomy surgery poses a number of unique challenges: many of the tissues involved are nearly transparent, the globe is relatively small in size, and special optical systems are necessary before a view is possible. Even then, some structures are optically inaccessible without special manipulation, and just a few drops of blood in the surgical field can completely obscure the view.
Improved visualization during vitrectomy therefore remains an ongoing quest for vitreoretinal surgeons. Several innovations have allowed this. Wide-angle viewing systems and the use of endoscopy during vitrectomy provide unfettered admission to areas that were previously inaccessible — namely the peripheral retina and vitreous base, wherein lies a vast amount of vitreoretinal pathology. The advent of smaller gauge, sutureless vitrectomy has also prompted the need for improved endoillumination through smaller instruments or from an additional fourth sclerotomy site. Tissue dyes are also employed to improve visualization and accurately remove thin and transparent tissue. One such use is staining of the internal limiting membrane (ILM) during macular hole repair and epiretinal membrane removal. Intraoperative hemorrhage can be a major problem by obscuring the view during vitrectomy.
Various pharmaceutical agents and instruments are used to decrease hemorrhage and improve visualization. For example, preoperative bevacizumab (Avastin, Genentech) is used to decrease intraoperative hemorrhage during tractional retinal detachment repair in proliferative diabetic retinopathy. Intraoperative thrombin and use of an end-aspirating endoilluminator (Alcon Laboratories, Fort Worth, Texas) also decrease bleeding. This article will review these modalities to facilitate visualization: wide angle viewing systems, endoillumination, tissue staining, and the use of pharmaceutical agents to promote intraoperative hemostasis. Both bevacizumab and thrombin are used here in an off-label capacity.
WIDE-ANGLE VITRECTOMY LENS SYSTEMS
Lens systems for vitrectomy initially employed planoconcave or biconcave lenses, which afforded only a 20� to 35� view.1 The retinal periphery was difficult to visualize, as even prism lenses could not provide visualization much past 60�.2 Wide-angle viewing systems were introduced and took advantage of the same principle on which the indirect ophthalmoscope is based: the astronomical telescope. In this system, the cornea and lens act together as the objective and the high-plus condensing lens as the eyepiece. This produces a real inverted aerial image of the retina and vitreous. However, performing bimanual surgery in an inverted fashion is counterintuitive for humans. Stereoscopic image inversion systems solve this problem typically with the use of 2 adjoining but orthogonally oriented prisms that can be moved into or out of the viewing path of the microscope3 in either a manual or mechanized manner.
Two types of wide-angle viewing systems for vitrectomy surgery exist: noncontact systems and contact systems. Each type has advantages and disadvantages. Contact systems typically provide a wider field of view with fewer aberrations and reflections. This is because the lens is directly coupled to the cornea via a viscous agent such as viscoelastic (eg, Healon, Advanced Medical Optics, Santa Ana, Calif) or hydroxypropyl methylcellulose (ie, Goniosol, Novartis). However, either a lens ring must be sewn on or a skilled assistant will be required to maintain the image quality. Contact lens systems include those produced by: Advanced Visual Instruments, Inc. (New York); Volk Optical, Inc. (Mentor, Ohio); and Ocular Instruments, Inc. (Bellevue, Wash).
Noncontact systems do not require a skilled assistant, and they cause less trauma to the corneal epithelium. Scleral depression is typically easier and manipulation of the microscope foot pedal remains consistent whether or not the image is inverted. The Insight Instruments binocular indirect ophthalmomicrosope (Stuart, Fla) employs a stereo inverter above the microscope objective, while the Moeller erect indirect binocular ophthalmic system, or (Hamburg, Germany), uses an inverter below the objective.
Wide-angle viewing systems have revolutionized vitrectomy procedures because surgeons now have access to the peripheral retina and vitreous base, the site of a vast amount of vitreoretinal pathology.
|
|
|
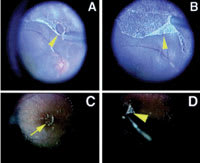
Figure 1. Triamcinolone acetonide assisted pars plana vitrectomy. A. The arrowhead shows that the posterior hyaloid can be separated from the retina by a silicone-tipped needle. B. The case of rhegmatogenous retinal detachment. C. The arrow shows that the island of the thin posterior hyaloid membrane stained with triamcinolone acetonide granulates on the macula. D. This membrane was removed by internal limiting membrane forceps (arrowhead) easily in the case of proliferative diabetic retinopathy. |
ILLUMINATION SYSTEMS
The introduction of wide-angle viewing systems was coupled with the development of more powerful light sources. While increased power allows for improved illumination, it is also associated with a higher risk of retinal phototoxicity. Retinal toxicity is thought to be secondary to photochemical exposure to shorter wavelength light, particularly ultraviolet and visible blue light.4 This can disrupt the blood-retinal barrier function and hence blue-light–blocking filters are used.5-7
Phototoxicity thresholds have been studied extensively to maximize
exposure time and minimize risk when developing light sources for vitrectomies.
Standard light sources available on a 20-gauge fiberoptic light probe include tungsten-halogen
and metal halide available as the Alcon Accurus (Fort Worth, Texas) and Bausch &
Lomb Millennium (Rochester, NY). These light sources typically have a maximum illumination
of 10 lumens, which is roughly halved when used with lighted instruments, wide-angle
probes, and chandelier systems through a standard 20-g light probe, leading to a
significant drop in illumination.8
The recent development of xenon light sources provides increased power output, with
increased illumination in smaller gauge probes and chandelier systems. To reduce
the risk of retinal phototoxicity, safety measures have been advocated. These include
the recommended use of a
475 μm long pass filter to block blue light, the
lowering of the power output, and perhaps most importantly, increasing the distance
between the light probe and the retina.9
Xenon light has been shown to have similar safety profiles in comparison to tungsten-halogen
and improved safety relative to metal halide light sources.10,11
One of the advantages of a xenon light source is the option to use it as an ancillary light in combination with wide-angle illuminators and instruments such as forceps or tissue manipulators. Another major advantage is that it can be used as the sole light source in a chandelier system, allowing bimanual surgery helpful in proliferative vitreoretinopathy (PVR) membrane dissection or peripheral and anterior vitreous base dissection (see Figure 6 in "Evolutionary and Revolutionary Trends in Vitreoretinal Surgery" by P Bhatnagar, HF Fine, and I-V Ho on p. 27). These also offer a wider angle of illumination, which is useful in 360-degree scleral depression. Given the increased working distance from the retina, this can significantly decrease retinal phototoxicity.9 Some currently available options include the Tornambe Torpedo (Insight Instruments, Buffalo, NY), the 25-g Awh Chandelier (Synergetics, O'Fallon, Mo), and fourth-port 25-g plug-in chandeliers such as the DORC Neptune Dual Chandelier (Kingston, NH).
Vitreoretinal Endoscopes
Wide-angle viewing systems and powerful light sources allow access to the far peripheral retina and ciliary body, but they often require scleral depression. Ophthalmic endoscopes can provide complete assessment of the pars plana in its natural state without scleral depression, and they can extend surgical control where standard microscopic views are limited. In complicated cases of proliferative vitreoretinopathy and iris neovascularization, where a small pupil, hyphema, or opaque posterior capsule can prevent complete visualization — even with standard and panoramic contact lenses — ophthalmic endoscopy can augment control in the retroirideal area.12 When used in conjunction with intraoperative fluorescein angiography, vitreoretinal endoscopy can identify pathologic areas in the pars plana and aid in intraoperative evaluation and further surgical treatment of complicated diabetic retinopathy.13,14 Endoscopy can also facilitate the management of complications of cataract surgery. It is used to visualize retained lens fragments that can be embedded in the vitreous base and to provide precise haptic placement in posteriorly dislocated intraocular lens implants.15,16 The learning curve can be steep as the surgeon's view is via television screen and therefore lacks stereopsis. However, new technology with high-definition televisions and 3-D software can now provide high-resolution stereoscopic views.17
VISUALIZATION AGENTS
|
|
|
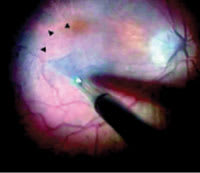
Figure 2. Operating microscope view demonstrating the continuous ILM peel in progress. A triangular flap of ILM is seen being peeled by forceps. Arrowheads indicate the area of ILM peeled in contrast with the unpeeled trypan blue stained ILM. |
Visualization of the ILM
Another advance in visualization during vitreoretinal surgery is the use of tissue dyes for selective staining of the ILM during removal. Various substances such as indocyanine green (ICG), trypan blue, and triamcinolone acetonide can be used. Note that all of these substances are used for this purpose off-label. Kadonosono et al reported the use of ICG to facilitate removal of the transparent ILM (see Figure 1 in "Evolutionary and Revolutionary Trends in Vitreoretinal Surgery" by P Bhatnagar, HF Fine, and I-V Ho on p. 25).18 Augmenting visibility of the ILM allows for effective and safe removal with less risk of retinal damage.
Many authors report positive outcomes in ILM removal with the use of ICG. Recent studies have shown equivalent anatomic and visual outcomes with and without the use of adjunctive ICG in ILM removal.19-24 Mavrofrides et al showed that ICG usage during macular hole surgery was not associated with worse visual outcome and that possible toxic side effects reported were not clinically significant.19 Kumagai et al reported decreased rates of macular hole reopening with ILM removal.21-22 However, the use of ICG has been controversial, given reports of toxicity to the retinal pigment epithelium (RPE). While the exact etiology of ICG-related retinal toxicity is unclear, experimental in vitro studies have suggested a cytotoxic mechanism with induced apoptosis of the RPE.25,26 Rabbit and mouse studies in vivo have been correlated with alterations in electroretinograms and histology.27-29 Clinical reports of poor visual acuity and visual field defects post-ICG application with ILM peeling were attributed to ICG-related toxicity.30-35 Notably, visual field defects have also been documented in cases of macular hole surgery without ILM removal.36
The deleterious effects of ICG appear to be compounded with increases in phototoxicity. Expression of apoptosis genes is upregulated in a dose-dependent manner when ICG is applied to human RPE cells. This effect was more pronounced with the direct application of endoillumination.27,37 While some argue that ICG phototoxicity is exacerbated most with light exceeding wavelengths of 620 μm,30 others noticed no change when applied in conjunction with a halogen light source for 15 minutes.38
Long-term intravitreal ICG in rat eyes caused ICG-related toxicity to retinal ganglion cells that was amplified when endoillumination was simulated.27 However, in this experimental model, the dye was left inside the eye for 1 month. This does not mirror clinical practice, in which ICG is typically applied for only 10 to 60 seconds and then immediately removed. With recent reports of persistence of intraocular ICG for anywhere from 1 to 8 months, this could become a potential source of concern.39-41 Given the continued dichotomy between theory and practice, the judicious use of ICG for ILM staining is currently advocated by many in ILM removal for macular hole surgery.
Triamcinolone Acetonide
Triamcinolone acetonide is a corticosteroid suspension that is commonly injected in an off-label fashion into the vitreous for the treatment of a wide variety of disorders, including retinovascular disease and macular degeneration. The crystals adhere to the vitreous gel, effectively highlighting the vitreous. This property can be extremely helpful in determining the location and attachments of the vitreous. For instance, intravitreal triamcinolone is useful while inducing a posterior vitreous separation intraoperatively or for highlighting peripheral vitreous when shaving the anterior vitreous base. Triamcinolone has been proposed as an alternative to ICG to enhance visualization of the internal limiting membrane.42-44 Triamcinolone is typically much less expensive than ICG and avoids toxicity to the RPE associated with ICG. Histological specimens collected at the time of removal confirm successful identification and delamination of the ILM with the use of triamcinolone.43 While it does not selectively stain the ILM, it improves visualization by dusting the ILM and providing a map for the surgeon to see what remains to be peeled (Figure 1). There is a large published experience with intravitreal triamcinolone in the literature, and well-documented side effects include steroid-induced glaucoma and cataract, typically at much higher concentrations than what remains after assistance for ILM peeling. A recent study using an in vivo rabbit model did not show histologic changes in the retina at triamcinolone concentrations of 0.5 mg or 1 mg, but it did note a dose-dependent retinal toxicity of the photoreceptor outer segments and RPE at higher triam-cinolone doses of 4 mg, 8 mg, and 20 mg.45
Trypan Blue
Trypan blue stains basement membranes and first gained widespread use in ophthalmology to stain the anterior lens capsule in preparation for capsulorhexis.46,47 Trypan blue has also successfully been used as an alternative dye to stain and assist in ILM and epiretinal membrane (ERM) removal (Figure 2).48 In vitro studies on human RPE cell lines have demonstrated no trypan blue dye related cell toxicity at concentrations as high as 1.5 mg/mL (stock solution). Clinically, doses are typically given at a concentration of 0.075 mg/mL (one-twentieth of stock solution).49 A retrospective study using trypan blue at a concentration of 0.06% during pars plana vitrectomy with ILM delineation and removal reported no toxic or adverse effects after 1 year.50
As it has become increasingly accepted that ILM removal improves anatomic and visual outcomes, new dyes have been investigated to facilitate ILM staining and removal while minimizing retinal toxicity. Brilliant blue G has been suggested as a safer alternative tissue dye.51 Haritoglou et al have evaluated novel vital dyes, such as light green SF yellowish, copper (II) phthalocyanine-tetrasulfonic acid, bromophenol blue, and Chicago blue, for intraocular surgery.52
MINIMIZING BLEEDING
Preoperative Bevacizumab to Decrease Intraoperative Hemorrhage
Intraoperative hemorrhage can be a major obstacle during vitrectomy for sequelae of proliferative diabetic retinopathy (PDR), such as tractional retinal detachment. Vascular endothelial growth factor (VEGF) has been implicated in the pathogenesis of PDR, and vitreous levels of VEGF are markedly elevated in PDR. Bevacizumab is a humanized murine monoclonal antibody that binds VEGF. Bevacizumab was developed and approved by the FDA for the treatment of metastatic colorectal cancer.53 Intravitreal bevacizumab has been used extensively to retard intraocular angiogenesis and vascular permeability. Early experience with intravitreal bevacizumab suggested that it might reduce hemorrhage associated with neovascularization. Intravitreal bevacizumab was injected in 2 patients with severe PDR complicated by vitreous hemorrhage and resulted in rapid regression of both the neovascularization and vitreous hemorrhage.54 Given these promising results, it was hypothesized that using bevacizumab preoperatively in patients with severe PDR and tractional retinal detachments could cause regression of neovascularization and decrease the risk of intraoperative hemorrhage.55 Bevacizumab was then given intravitreally 2 weeks preoperatively with significant regression of neovascularization seen on fundus photography.
Preoperative regression of neovascularization allows for more straightforward removal of fibrotic tractional membranes with minimal bleeding during segmentation and delamination.
Intravitreal Thrombin
Another adjuvant used to minimize intraoperative hemorrhage is intravitreal thrombin. In vivo and clinical studies have shown a significant reduction in both bleeding time after cutting vascularized membranes and overall rate of intra- and postoperative bleeding.56,57 Toxicity studies show no effect on the lens, corneal endothelium, or visual acuity.58,59 Electroretinograms demonstrated normal b-wave amplitudes, but decreased sensitivity has been noted.60 Given the lack of toxicity, postoperative hemostasis can be maintained for up to 1 week if the intravitreal thrombin is not washed out.59
CONCLUSIONS
Improved visualization during vitrectomy surgery is one of the major factors that has improved surgical outcomes and allowed retinal physicians to approach more and more difficult cases. Wide-angle viewing has afforded surgeons a three-dimensional intraoperative view of the peripheral retina, vitreous base, ora serrata, pars plana and pars plicata, and ciliary body. Illumination systems have evolved along with smaller-gauge surgery, and newer light sources allow for bimanual surgery, wider-angle viewing to visualize anterior ocular structures, and improved contrast for detecting subtle membranes and the ILM. Ophthalmic endoscopy can provide complete assessment of the retroirideal space in its natural state without scleral depression and extend surgical control where standard microscopic views are limited. Tissue-staining techniques have provided methods to better illuminate vitreous gel, aiding in posterior vitreous separations and anterior vitreous shaving, as well as highlighting membranes and the ILM to improve the ability of surgeons to perform peeling procedures. Finally, therapeutics designed to reduce intraoperative bleeding simplify surgical cases and improve outcomes. Future advances in the field of vitrectomy surgery will undoubtedly include further improvements in visualization.
REFERENCES
1. Landers MB 3rd, Stefansson E, Wolbarsht ML. The optics of vitreous surgery. Am J Ophthalmol. 1981;91:611-614.
2. Bovey EH, Gonvers M. A new device for noncontact wide-angle viewing of the fundus during vitrectomy. Arch Ophthalmol. 1995;113:1572-1573.
3. Chalam KV, Shah VA. Optics of wide-angle panoramic viewing system-assisted vitreous surgery. Surv Ophthalmol. 2004;49:437-445.
4. Ham WT, Mueller HA, Ruffolo JJ et al. Action spectrum for retinal
injury near ultraviolet radiation in the aphakic monkey. Am J Ophthalmol.
1982;93:
299-306.
5. Van Best JA, Putting BJ, Oosterhuis JA, et al. Function and morphology of the retinal pigment epithelium after light-induced damage. Microsc Res Tech. 1997;36:77-88.
6. Putting BJ, Van Best JA, Vrensen GF, et al. Blue-light-induced dysfunction of the blood-retinal barrier at the pigment epithelium in albino versus pigmented rabbits. Exp Eye Res. 1994;58:31-40.
7. Putting BJ, Zweypfenning RC, Vrensen GF, et al. Blood-retinal barrier dysfunction at the pigment epithelium induced by blue light. Invest Ophthalmol Vis Sci. 1992;33:3385-3393.
8. Chow DR. Shedding some light on current endoillumination. Retinal Physician. 2005;2:37-39.
9. Van den Biesen PR, Berenschot T, Verdaasdonk RM, et al. Endoillumination during vitrectomy and phototoxicity thresholds. Br J Ophthalmol. 2000;84:1372-1375.
10. Data on file, Alcon Research Ltd.
11. Haritoglou C, Priglinger S, Gandorfer A, et al. Histology
of the vitreoretinal interface after indocyanine green staining of the ILM, with
illumination using a halogen and xenon light source. Invest Ophthalmol Vis Sci.
2005;46:
1468-1472.
12. Ciardella AP, Fisher YL, Carvalho C, et al. Endoscopic vitreoretinal surgery for complicated proliferative diabetic retinopathy. Retina. 2001;21:20-27.
13. Terasaki H, Miyake Y, Awaya S. Fluorescein angiography of peripheral retina and pars plana during vitrectomy for proliferative diabetic retinopathy. Am J Ophthalmol. 1997;123:370-376.
14. Uram M. Endoscopic fluorescein angiography. Ophthalmic Surg Lasers. 1996;27:849-855.
15. Chan CK, Agarwal A, Agarwal S. Management of dislocated intraocular implants. Ophthalmol Clin North Am. 2001;14:681-693.
16. Boscher C, Lebuisson DA, Lean JS, et al. Vitrectomy with endoscopy for management of retained lens fragments and/or posteriorly dislocated intraocular lens. Graefes Arch Clin Exp Ophthalmol. 1998;236:115-121.
17. Miyake K, Ota I, Miyake S, et al. Application of a newly developed, highly sensitive camera and a 3-dimensional high-definition television system in experimental ophthalmic surgeries. Arch Ophthalmol. 1999;117:1623-1629.
18. Kadonosono K, Itoh N, Uchio E, et al. Staining of internal limiting membrane in macular hole surgery. Arch Ophthalmol. 2000;118:1116-1118.
19. Mavrofrides E, Smiddy WE, Kitchens JW, et al. Indocyanine green-assisted internal limiting membrane peeling for macular holes: toxicity? Retina. 2006;26:637-644.
20. Kwok AK, Lai TY, Yuen KS, et al. Macular hole surgery with or without indocyanine green stained internal limiting membrane peeling. Clin Experiment Ophthalmol. 2003;31:470-475.
21. Kumagai K, Furukawa M, Ogino N, et al. Long-term outcomes of internal limiting membrane peeling with and without indocyanine green in macular hole surgery. Retina. 2006;26:613-617.
22. Kumagai K, Furukawa M, Ogino N, et al. Vitreous surgery with and without internal limiting membrane peeling for macular hole repair. Retina. 2004;24:721-727.
23. Da Mata AP, Burk SE, Riemann CD, et al. Indocyanine green-assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for macular hole repair. Ophthalmol. 2001;108:1187-1192.
24. Kwok AK, Lai TY, Man-Chan W, et al. Indocyanine green assisted retinal internal limiting membrane removal in stage 3 or 4 macular hole surgery. Br J Ophthalmol. 2003;87:71-74.
25. Sippy BD, Engelbrecht NE, Hubbard GB, et al. Indocyanine green effect on cultured human retinal pigment epithelial cells: implication for macular hole surgery. Am J Ophthalmol. 2001;132:433-435.
26. Rezai KA, Farrokh-Siar L, et al. Indocyanine green induces apoptosis in human retinal pigment epithelial cells. Am J Ophthalmol. 2004;137:931-933.
27. Yip HK, Lai TY, So KF, et al. Retinal ganglion cells toxicity caused by photosensitising effects of intravitreal indocyanine green with illumination in rat eyes. Br J Ophthalmol. 2006;90:99-102.
28. Kwok AK, Lai TY, Yeung CK, et al. The effects of indocyanine green and endoillumination on rabbit retina: an electroretinographic and histological study. Br J Ophthalmol. 2005;89:897-900.
29. Goldstein M, Zemel E, Loewenstein A, et al. Retinal toxicity of indocyanine green in albino rabbits. Invest Ophthalmol Vis Sci. 2006;47:2100-2107.
30. Gandorfer A, Haritoglou C, Gandorfer A, et al. Retinal damage from indocyanine green in experimental macular surgery. Invest Ophthalmol Vis Sci. 2003;44:316-23.
31. Haritoglou C, Gandorfer A, Gass CA, et al. Indocyanine green-assisted peeling of the internal limiting membrane in macular hole surgery affects visual outcome: a clinicopathologic correlation. Am J Ophthalmol. 2002;134:836-41.
32. Horio N, Horiguchi M. Effect on visual outcome after macular hole surgery when staining the internal limiting membrane with indocyanine green dye. Arch Ophthalmol. 2004;122:992-6.
33. Gandorfer A, Haritoglou C, Gass CA, et al. Indocyanine green-assisted peeling of the internal limiting membrane may cause retinal damage. Am J Ophthalmol. 2001;132:431-433.
34. Ando F, Sasano K, Ohba N, et al. Anatomic and visual outcomes after indocyanine green-assisted peeling of the retinal internal limiting membrane in idiopathic macular hole surgery. Am J Ophthalmol. 2004;137:609-614.
35. Uemura A, Kanda S, Sakamoto Y, et al. Visual field defects after uneventful vitrectomy for epiretinal membrane with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2003;136:252-257.
36. Ezra E, Gregor ZJ; Morfields Macular Hole Study Group Report No. 1. Surgery for idiopathic full-thickness macular hole: two-year results of a randomized clinical trial comparing natural history, vitrectomy, and vitrectomy plus autologous serum: Moorfields Macular Hole Study Group Report no. 1. Arch Ophthalmol. 2004;122:224-236.
37. Yam HF, Kwok AK, Chan KP, et al. Effect of indocyanine green and illumination on gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:370-377.
38. Iriyama A, Uchida S, Yanagi Y, et al. Effects of indocyanine green on retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:943-947.
39. Spaide RF. Persistent intraocular indocyanine green staining after macular hole surgery. Retina. 2002;22:637-639.
40. Ashikari M, Ozeki H, Tomida K, et al. Retention of dye after indocyanine Green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2003;136:172-174.
41. Ciardella AP, Schiff W, Barile G, et al. Persistent indocyanine
green fluorescence after vitrectomy for macular hole. Am J Ophthalmol. 2003;136:
174-177.
42. Horio N, Horiguchi M, Yamamoto N. Triamcinolone-assisted internal limiting membrane peeling during idiopathic macular hole surgery. Arch Ophthalmol. 2005;123:96-9.
43. Tognetto D, Zenoni S, Sanguinetti G, et al. Staining of the
internal limiting membrane with intravitreal triamcinolone acetonide. Retina.
2005;25:
462-467.
44. Shah GK, Rosenblatt BJ, Blinder KJ, et al. Triamcinolone-assisted internal limiting membrane peeling. Retina. 2005;25:972-975.
45. Yu SY, Damico FM, Viola F, et al. Retinal toxicity of intravitreal triamcinolone acetonide: a morphological study. Retina. 2006;26:531-536.
46. Melles GR, de Waard PW, Pameyer JH, et al. Trypan blue capsule staining to visualize the capsulorhexis in cataract surgery. J Cataract Refract Surg. 1999;25:7-9.
47. Norn MS. Perioperative trypan blue vital staining of corneal endothelium. Eight years' follow up. Acta Ophthalmol (Copenh). 1980;58:550-555.
48. Feron EJ, Veckeneer M, Parys-Van Ginderdeuren R, et al. Trypan blue staining of epiretinal membranes in proliferative vitreoretinopathy. Arch Ophthalmol. 2002;120:141-144.
49. Gale JS, Proulx AA, Gonder JR, et al. Comparison of the in vitro toxicity of indocyanine green to that of trypan blue in human retinal pigment epithelium cell cultures. Am J Ophthalmol. 2004;138:64-69.
50. Perrier M, Sebag M. Trypan blue-assisted peeling of the internal limiting membrane during macular hole surgery. Am J Ophthalmol. 2003;138:903–905.
51. Haritoglou C, Tadayoni R, May CA, et al. Short-term in vivo evaluation of novel
vital dyes for intraocular surgery. Retina. 2006;26:673-678.
52. Enaida H, Hisatomi T, Hata Y, et al. Brlliant Blue G selectively stains the internal limiting membraine/brilliant blue G-assisted membrane peeling. Retina 2006;26:631-636.
53. Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391-400.
54. Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275-278.
55. Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. 2006;26:699-700.
56. Thompson JT, Glaser BM, Michels RG, et al. The use of intravitreal thrombin to control hemorrhage during vitrectomy. Ophthalmol. 1986;93:279-282.
57. Glaser BM. The use of intravitreal thrombin to control hemorrhage during retinectomy. Retina.1988;8:1-2.
58. DeBustros S, Glaser BM, Johnson MA. Thrombin infusion for
the control of intraocular bleeding during vitreous surgery. Arch Opthalmol.
1985;103:
837-839.
59. Kim SH, Cho YS, Choi YJ. Intraocular hemocoagulase in human vitrectomy. Jpn J Ophthalmol.1994;38:49-55.
60. Kim SH, Lee HS, Kim IT, et al. Efficacy of intravitreal hemocoagulase for control of bleeding in rabbit experimental model of vitrectomy. Jpn J Ophthalmol.1993;37:379-384.
Kristie L. Lin, MD, practices ophthalmology at the St. Vincent Catholic Medical Center in New York. Howard F. Fine, MD, MHSc, is a postdoctoral clinical fellow with the Harkness Eye Institute of the Columbia University College of Physicians and Surgeons in New York and practices ophthalmology at Vitreous Retina Macula Consultants of New York. Neither author has any financial interest in any of the information contained in this article. Dr. Lin can be contacted at (212) 604-8041.