PEER REVIEWED
Managing
Intraocular Pressure Elevation Following Intravitreal Steroid Injection
ROBERT J. NOECKER, MD, MBA
The use of intravitreal steroids as a treatment for cystoid macular edema, macular edema from vascular diseases, proliferative retinopathy, choroidal neovascularization from age-related macular degeneration (AMD), and other retinal disorders has become an increasingly popular treatment modality.1-4
However, it is not uncommon for patients who have had intravitreal triamcinolone acetonide (Kenalog, Bristol-Myers Squibb) (IVTA) injections to suffer adverse events such as secondary open-angle glaucoma or cataract formation or progression.
The intraocular administration of corticosteroids has the benefit
of delivering high concentrations of drug while minimizing ocular surface or systemic
side effects (Figure 1). The trade-off is the risk of developing elevated intraocular
pressure (IOP), which is the most
common of complications. Other less common,
yet vision-threatening complications, include endophthalmitis, vitreous hemorrhage,
retinal detachment, progressive cataract formation, and secondary ocular hypertension.
Although a secondary IOP rise is possible with any steroid administration, the risk associated with intravitreal injection appears to be higher due to the prolonged (and perhaps repeated) elevated concentrations that are achieved intraocularly.
In addition, the subsequent risk of chronic glaucoma is not yet clear. While most patients with IOP elevations post-IVTA injection are treated successfully with topical medications, a small percentage of patients require filtration surgery to lower IOP.2,5 This article will investigate the mechanisms that trigger IOP elevation after IVTA injections and offer treatment strategies when monitoring such complications.
|
|
|
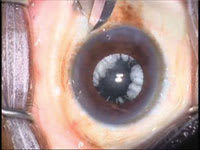
Figure 1. Injection of IVTA through pars plana with the drug visible as white particles behind the IOL. |
PROBLEM RECOGNITION
The first step in managing steroid-induced glaucoma is recognizing the condition. It has been well known for over 50 years that localized therapy with corticosteroids can result in IOP elevation.6 Typical features of the clinical presentation are an IOP rise within a few weeks after beginning potent topical corticosteroids and within a few months with weaker ones. In some patients, particularly those with open-angle glaucoma, the IOP rise can be much more acute and occur within hours after administration.
In the general population, the majority of patients tolerate short-term topical steroid exposure well. In the historical literature, 5%-6% of normal individuals have developed IOP spikes after 4-6 weeks of topical dexamethasone or betamethasone therapy. Traditionally, steroid use has been avoided in patients at risk for IOP spikes. Patients with open-angle glaucoma and ocular hypertension have been shown to have much higher response rates to steroids. The rate has been reported to be as high as 90% in patients with open-angle glaucoma receiving topical 0.1% dexamethasone for 4 weeks.7 Those also at higher risk include those with a family history of the disease, high myopes, those with rheumatologic diseases, and younger patients.
IOP ELEVATION
The steroid-associated IOP elevation has been reported to be due to a reduction in the facility of aqueous outflow.7,8 Trabecular and anterior uveal meshwork tissue have a high concentration of corticosteroid receptors.9 The mechanism of action for IOP elevation may be an increase in extracellular matrix proteins, a decrease in the phagocytic activity of the trabecular endothelial cells, and a decrease in prostaglandins that regulate outflow.10-12
Acutely, intravitreal injections may cause IOP elevation due to mechanical obstruction of the trabecular meshwork by the steroid particles or its vehicle. An early and rapid rise in IOP within 1 week associated with white material within the angle after an intravitreal triamcinolone injection has been reported.13 However, in most cases, no material is detectable. Alternatively, patients who experience acute IOP elevations may have a reduced facility of outflow that cannot compensate for any increased demand on outflow due to the increased pressure in the eye following injection.
The pressure-inducing effect of steroids are related to the anti-inflammatory potency of the particular drug as well as the duration of exposure. The prolonged therapeutic window of triamcinolone acetonide appears to be due to its minimal water solubility. In 1 study, the average half-life of the drug was found to be 18.6 days following intravitreal injection of 4 mg in 0.1-cc triamcinolone in non-vitrectomized human eyes. The drug was measurable in the vitreous for up to 3 months.14
An IOP elevation following intravitreal triamcinolone acetonide injection, if it occurs, will typically be diagnosed during the first 2 months post-injection. Jonas et al recorded the IOP response in 75 eyes of patients diagnosed with exudative AMD or diabetic macular edema who received a 25-mg injection of intravitreal triamcinolone.1 After an average of 2 months following the 25-mg injection, 52% of patients experienced an IOP rise to 21 mm Hg or greater. In this report, all eyes except for 1 were controlled on topical medication. In that 1 remaining case, a patient with known pre-injection glaucoma went on to have filtration surgery to control the pressure.
In a safety study of the lower dose of 4-mg intravitreal triamcinolone, the duration of IOP elevation persisted for at least 6 months in most eyes, and the mean duration of treatment of corticosteroid-induced IOP elevation was 8 months.15 In another study, a single 4-mg intravitreal triamcinolone acetonide injection was associated with an increase in IOP of 10 mm Hg or greater in 27.9% of eyes. Smithen et al reported a 40% incidence of IOP elevation to greater than 24 mm Hg3 after a mean of 100.6 days post-injection. They also reported a significant relationship between preoperative IOP and likelihood of steroid response. Patients without glaucoma and baseline pressures less than 15 mm Hg, were less likely to experience IOP spikes above 24 mm Hg (P<.01). Twelve of the 89 (13.5%) patients were known to have glaucoma prior to IVTA injection and most patients (80.9%) were diagnosed with AMD.
These data suggest that a significant incidence of moderate-to-severe elevation of IOP may occur following intravitreal triamcinolone injection. Although the IOP elevations are controllable with medical therapy in most patients, the risk of intractable secondary glaucoma necessitating surgical intervention does exist but has so far been discussed infrequently. There is limited information in the literature about surgical outcomes in this group alone.
STEROID-INDUCED GLAUCOMA
|
|
| Figure 2. Macular OCT demonstrates patient had extensive cystoid changes and edema. |
Management of steroid-induced glaucoma centers around discontinuation of the drug. In the case of IVTA, this may mean a vitrectomy to remove the drug if the IOP elevation is very high and the optic nerve is felt to be susceptible to damage in a short period of time.
While the response to the initial IVTA injection can often be a good predictor about the response to future injections, IOP should be continuously monitored as an IOP spike is still possible during subsequent IVTA treatments.
For example, although a patient might be more likely to develop an elevated IOP immediately following the first injection, it can still happen after a subsequent IVTA treatment. For patients who maintain an elevated IOP for several weeks, further steroid injections should be avoided until the IOP is controlled.
When deciding how best to manage the IOP elevation, much depends on the status of the patient's optic nerve prior to treatment. That is, the healthier the optic nerve is, the better tolerated increases in IOP will be. If a patient has a normal optic nerve, visual field, and retinal nerve fiber layer, then an IOP of 30 mm Hg for several weeks is unlikely to be devastating. Conversely, if the patient already has some optic nerve damage or compromise, then more damage is likely to occur.
Using corneal pachymetry can also guide tolerance for increased IOP. An IOP reading of 30 mm Hg in a patient with a 600 μm cornea may be an overestimation that is not much higher than the patient's baseline IOP. On the other hand, a patient with a thin, 480 μm cornea who develops an IOP of 30 mm Hg is probably at much greater risk for developing optic nerve damage and should be treated.16
Whether or not to enlist the assistance of a glaucoma specialist is one of practitioner comfort. The more comfortable one is in evaluating the appearance of the optic nerve, nerve fiber layer, and visual field, the less added benefit will come from the consultation. However, if the retinal specialist chooses to manage the patient alone, the optic nerve, nerve fiber layer, and visual field should be documented early on in the process to determine the baseline status of these parameters.
While typical glaucomatous changes take months to years to manifest themselves, there are some early signs that more aggressive therapy may need to undertaken. If an optic-disc hemorrhage is seen, this is likely a sign of progression and may indicate that more aggressive therapy is necessary. If an HRT2 or an OCT is available, an image of the optic nerve or nerve fiber layer can be obtained to detect any baseline abnormalities. A stereoscopic photograph of the optic disc is also helpful for baseline documentation of the optic nerve and nerve fiber layer. Visual fields sometimes have limited utility if the central vision is significantly compromised from the macular condition.
|
|
|
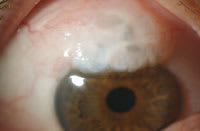
Figure 3. The patient developed a functioning filtering bleb following trabeculectomy. |
SURGICAL OPTIONS
There are no published studies demonstrating the superiority of 1 medical or surgical treatment over another for steroid-associated glaucoma. Medical management of this condition often follows the same treatment premise of traditional treatment for open-angle glaucoma.
Typically, both aqueous suppressants such as brimonidine tartrate 0.15% (Alphagan P, Allergan), timolol 0.5% (Timoptic, Merck), and a topical carbonic anhydrase inhibitor (CAI) are used. Outflow agents such as 1 of the prostaglandin analogues are also used if there is not concern about exacerbating macular edema. Systemic CAIs may also be used with some caution about systemic side effects. The most common side effects encountered include parathesias, nausea, depression, and renal stones.
Laser trabeculoplasty is a treatment alternative or adjunct to medical therapy. A recent study by Viola and colleagues evaluated the role of argon laser trabeculoplasty (ALT) in treating intractable glaucoma post-IVTA injection.17 Anecdotally, selective laser trabeculoplasty (SLT) appears to work similar to ALT.
Surgical therapy can be utilized in cases insufficiently responsive to medical or laser therapy in a timely fashion. Trabeculectomy can be performed in those cases without significant conjunctival scarring from prior surgery. In fact, the success rate may be higher in these eyes due to the depot effect of the steroid in the eye which can slow fibroblast proliferation in the conjunctiva.
If there is significant scarring or inflammation, then a glaucoma drainage device such as a Molteno, Ahmed, or Baerveldt may be used. The Ahmed may begin reducing the IOP sooner, as the others typically need temporally ligation before opening because they lack valves.
CASE REPORT
A 40-year-old diabetic woman presented with the chief complaint of progressive decreased vision in the left eye. Presenting vision was 20/30 OD and 20/200 OS and IOP was 15 mm Hg OU. Slit lamp examination showed a normal anterior segment and angle by gonioscopy in each eye. Fundus examination revealed a cup:disc ratio of 0.5 OU and clinically significant diabetic macular edema OS refractory to prior focal laser treatment (Figure 2). To treat the macular edema, 4 mg/0.1 ml of triamcinolone acetonide was injected intravitreally. One month later, the visual acuity improved 20/60 but the IOP was noted to be 22 mm Hg OD and 32 mm Hg OS. Timolol 0.5% was started to lower the pressure. Eight weeks after the injection, the IOP was 20 mm Hg OD and 42 mm Hg OS despite the addition of brimonidine tartrate 0.15, oral acetazolamide (Diamox Sequels, Barr Laboratories) and topical latanoprost (Xalatan, Pfizer). Because the patient's IOP was not well controlled, the patient underwent trabeculectomy with mitomycin-C (Figure 3). Six months postoperatively, visual acuity was 20/30 OD and 20/60 OS, and the IOP was 15 mm Hg OD and 14 mm Hg OS.
COMANAGEMENT
In terms of follow-up, it may be beneficial for patients with pre-existing glaucoma to be comanaged with a glaucoma specialist because there is less room for error in these patients with compromised optic nerves. Patients considered at risk should have an IOP measurement in both eyes within 1 week of the initial injection and then several weeks later if the IOP is acceptable.
CONCLUSION
The occurrence of intractable elevated IOP and secondary glaucoma is a serious complication after IVTA injection. The risks of this potentially devastating complication need to be weighed against the benefits of intravitreal triamcinolone acetonide in the individual patient. A high index of suspicion and early recognition of the condition can lead to better outcomes and faster resolution of the elevated IOP with preservation of the optic nerve and visual field.
REFERENCES
1. Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003;87:24-27.
2. Jonas JB, Kreissig I, Degenring RF. Secondary chronic open-angle glaucoma after intravitreal triamcinolone acetonide. Arch Ophthalmol. 2003;121:729-730.
3. Smithen LM, Ober MD, Maranan L, et al. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004;138:740-743.
4. Bashshur ZF, Ma'luf RN, Allam S, et al. Intravitreal triamcinolone for the management of macular edema due to nonischemic central retinal vein occlusion. Arch Ophthalmol. 2004;122:1137-1140.
5. Park CH, Glenn JJ, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. Am J Ophthalmol. 2003;136:419-425.
6. Francois J. Cortisone et tension oculare. Ann D'Oculist. 187;805:1954.
7. Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. II. The effect of dexamethasone in the glaucomatous eye. Arch Ophthalmol. 1963;70:492.
8. Mccarty GR, Schwartz B. Increased concentration of glucocorticoid receptors in rabbit iris-ciliary body compared to rabbit liver. Invest Ophthalmol Vis Sci. 1982;23:525.
9. Southern AL, Gordon GG, L'hommedieu D. 5B-dihyrocortisol: possible mediator of the ocular hypertension in glaucoma. Invest Ophthalmol Vis Sci. 1985;26:393.
10. Spaeth GL, Rodrigues MM, Weinreb S. Steroid-induced glaucoma: A. Persistent elevation of intraocular pressure. B. Histopathologic aspects. Trans Am Ophthalmol Soc. 1977;75:353-381.
11. Johnson DH, Bradley JMB, Accott TS. The effect of dexamethasone on glycosoaminoglycans of human trabecular meshwork in perfusion organ culture. Invest Ophthalmol Vis Sci. 1990;31:2568.
12. Seftor REB, Stamer WD, Seftor EA, Snyder RW. Dexamthasone decreases tissue plasminogen activator activity in trabecular meshwork organ and cell cultures. J Glaucoma. 1994;3:323.
13. Singh IP, Ahmad SI, Yeh D, Challa P, Herndon LW, Allingham RR, Lee PP. Early rapid rise in intraocular pressure after intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2004;138:286-287.
14. Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB 3rd, Miller M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110:681-686.
15. Gillies MC, Simpson JM, Billson FA, et al. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004;122:336-340.
16. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714-720.
17. Viola F, Morescalchi F, Staurenghi G. Argon laser trabeculoplasty for intractable glaucoma following intravitreal triamcinolone. Arch Ophthalmol. 2006;124:133-134.
Robert Noecker, MD, is vice chair of the Department of Ophthalmology at the University of Pittsburgh. He is also director of the Glaucoma Service. He has no financial interest in any of the information contained in this article Dr. Noecker can be e-mailed noeckerrj@UPMC.EDU.