PEER
REVIEWED
Macular
Hole Update: 2006
WILLIAM
E. SMIDDY, MD
The topic of macular holes has matured from solely academic interest to being centered on therapeutics. Better imaging capabilities offered by optical coherent tomography (OCT) allow more accurate diagnosis especially at early stages, shed light on their pathogenesis, and may predict or explain postoperative visual results. Interest in understanding the pathogenesis of macular holes continues, but the focus is making the surgical success rate higher and more easily achievable for the patient. Specifically, the intraoperative technique of internal limiting membrane (ILM) peeling, and the regimen of postoperative positioning have been examined.
|
|
|
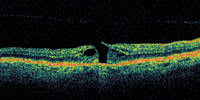
Figure 1. OCT showing early macular hole formation. The lateral separation is less than 50 μm. |
|
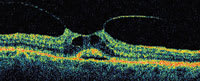
| Figure 2. There is subretinal fluid beginning to form under the central fovea in what may represent a prehole condition. No full-thickness defect can be observed in any cut. |
|
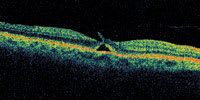
| Figure 3. This patient has vision of 20/30, but has an early apparent peeling of the inner retina along one side of the fovea. This may represent a laterally ruptured cyst and may or may not represent an early macular hole formation. |
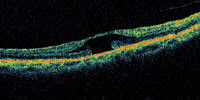
|
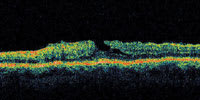
| Figure 4. A patient with an irregular shape to the fovea. This may represent a lamellar macular hole or a self-healed full-thickness macular hole. |
PATHOGENESIS
The grading scheme introduced by Gass represents an appropriate framework upon which to build observations and ideas from many investigators, and interpretation of several imaging modalities,1-3 but pathogenesis is still incompletely understood. A coup-contrecoup force mechanism of formation has been presumed for traumatically induced macular holes, even though some holes form days or weeks after trauma.4 Possibly, an intermediate step involves cystoid changes which gradually consolidate and rupture.5 This might parallel what may happen in idiopathic macular hole formation — the breakdown of large, consolidated foveal cysts. The development of techniques such as slit-lamp biomicroscopy and fluorescein angiography allowed reliable distinction between the cystic changes seen in conditions such as macular holes and the cystoid macular edema (CME) seen in inflammation-associated conditions.6 However, the subtleties confounding accurate distinction of macular hole, impending macular hole, and pseudohole conditions are now well recognized.
The steps inducing the cyst and its breakdown are more abstruse. A firm vitreoretinal adhesion at the fovea and optic disc was demonstrated by clinicopathologic correlation, first bolstering the evidence for a traction-induced cause.7 Subsequent clinical series implicating the completion of a posterior vitreous detachment (PVD) abounded, seemingly solidifying traction as the major event, at least in the majority of cases.8
The first comprehensive study of the histopathology of macular holes included 3 major findings that might carry implications for pathogenesis.9 First, at least a partial posterior vitreous detachment was observed, but direct vitreoretinal connections were absent near the hole so no traction was inferred. Second, cystoid changes were typically more marked in the outer retina than the inner retina. Third, pigment demarcation delimiting the small area of serous elevation around the edges of the macular hole was present in some eyes. Secondary findings included partially separated internal ILM and opercula in several cases. A subsequent histopathologic study published approximately 10 years later from the same laboratory reported 12 of 22 full-thickness macular holes with vitreous adherent to a detached tissue plug (operculum) suspended over the hole, but no agent of tangential vitreous traction was identified in the full-thickness cases.10 The authors' conclusion was that epiretinal membrane traction might play a role in the formation of full-thickness macular holes.
Three histopathologic studies of eyes in which macular hole surgery
was successful in closing a macular hole have been reported.11-13
Collectively, these reports noted re-approximation of Mueller cells (and glial cells),
normal-appearing underlying retinal pigment epithelium (RPE),
a lack of inflammatory
response, an apparent resolution of CME, and, interestingly, a lack of ILM around
the margins of the macular hole in some cases, despite no purposeful attempts to
peel that layer at the time of surgery. The authors' consensus was that the holes
closed because removal of cortical vitreous allowed relief of tangential traction
and permitted re-approximation of the edges. They observed that while some photoreceptor
loss must have occurred during the process of macular hole formation, the array
of relatively undisturbed photoreceptors at the area of re-approximation was surprising,
yet consistent with the fairly high degree of visual improvement often obtained
after macular hole surgery.
Two electron micrographic studies of apparent opercula collected at the time of macular hole surgery came to opposing conclusions.14-15 One found the opercula in 2 eyes to consist of proliferated fibrous astrocytes and Mueller cells, emphasizing the absence of distinct retinal neuronal tissue and calling into question the retinal nature of the operculum.14 The other interpreted the finding of outer retinal, neuronal elements in 7 of 18 specimens and concluded opercula are full-thickness retinal plugs in at least many cases.15 This conclusion was supported by their subsequent study utilizing immunochemical staining techniques that found photoreceptor cell elements in 8 of 12 specimens.16 The authors found poorer anatomic results in those cases in which the operculum included photoreceptor elements and hypothesized that the variable degree of retinal elements in the operculum reflects the variable depth of inner retinal cleavage during hole formation, and probably correlates with the degree of anatomic and visual success following macular hole surgery. Inherent to this hypothesis is the conclusion that the extent of foveal damage may be largely predetermined when the macular hole forms and, therefore, limits visual recovery regardless of surgical technique.
Another histopathologic study acknowledged the dilemma of determining whether the glial proliferation was primary and caused the hole, or whether it was a secondary reaction.17
The success of macular hole surgical techniques stimulated a resurgence in understanding the normal anatomy of the vitreoretinal interface. The vitreous body structure is substantially more complex than it first appears; it is not a simple, homogenous structure. Cisternal spaces within the vitreous body have been described long ago,18 and when exaggerated probably correlate to the vitreoschisis cavities observed clinically,19,20 histologically,21 and echographically.22 Such an anatomic feature may be the basis for commonly encountered persistent cortical vitreous attachment and provide the conduit for tangential traction. Attached vitreous remnants demonstrated by scanning electron microscopic study in the perifoveal area in eyes with apparent posterior vitreous detachment suggest that posterior vitreous separation may also not be as simple and "clean" as intuition suggests.23 These persistent elements may also provide the substrate for surface traction.
The ILM is attenuated to the point of discontinuity at the central fovea.24 Henle's nerve fiber layer, the inverted cone of outer plexiform layer (including Mueller cells) at the fovea, may be a key site of macular hole initiation since both Henle's layer and the Mueller cells assume a more oblique orientation in the central fovea (the umbo), rendering them more structurally vulnerable to tangential, shearing forces and may proliferate to populate the operculum or complete the hole formation.24-25 Cyst formation may be a preliminary reflection or consequence of such forces.26
The concept of a lamellar macular hole, introduced long ago, was postulated to occur from rupturing of the inner wall cysts of a patient with CME.27 Apparent histopathologic correlates were studied.9,10
Reports of impending macular holes (Stage 1A) described foveal cystic changes and, in some cases, a central consolidated cyst that may have represented a coalescence of previous microcysts.28-30 Although preliminary studies of surgery to prevent progression to full-thickness macular holes suggested efficacy,31,32 a prospective study of vitrectomy for impending macular holes did not confirm these findings, possibly due to underrecruitment.33
THE ROLE OF OCT
|
|

|
| Figure 5. A vitreofoveal traction (top) which was apparently representing a pre-macular hole condition presented. The patient's vision was 20/50. Surgery was scheduled, but upon returning for a follow-up visit preoperatively approximately 3 weeks later the visual acuity had improved to 20/20. The follow-up OCT (bottom) shows resolution of the traction with no defect of the macular hole. |
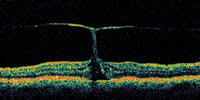
|
| Figure 6. This patient has very prominent vitreofoveal attachment. There is apparently proliferation of cells along the still attached strand of hyaloid with some proliferation along the posterior hyaloid margins. The patient did develop a full-thickness macular hole in both eyes during the course of follow-up. |
A central clinical challenge has been accurately diagnosing early macular holes which may be mimicked by many other conditions.34,35 No imaging modality has enhanced our diagnostic capability more than the OCT; superior imaging capabilities have improved diagnostic accuracy, clinical monitoring, and postoperative assessment.36-38 The OCT unequivocally demonstrates early stages of full-thickness macular holes (Figure 1), and allows distinction of pseudohole and pre-macular hole conditions in almost all instances. Fluid accumulation in early, presumed pre-macular hole stages have been corroborated by OCT observations (Figure 2). Serial images have been reported showing the progression from apparent impending macular holes to full thickness macular holes.39-42 It has depicted many other configurations that might be in the spectrum of lamellar or pre-macular hole conditions (Figure 3 and 4).
Serial OCT exams shed more light on probable pathogenesis and clinical staging. Resolution of the foveal cystic change/impending macular holes after posterior cortical vitreous release (aborted Stage 1B) is readily imaged using OCT (Figure 5).43 The OCT has documented spontaneous closure (without high vitreous separation) of several traumatic macular holes44-47 and also an idiopathic macular hole without48 and with high vitreous separation.49,50
Optical coherence tomography renderings of impending holes are reminiscent of the early reports by Reese which initially suggest vitreofoveal traction as a macular hole precursor.51,52 While sometimes the OCT images seem to support that hypothesis, static images and clinical observations do not depict force lines or subsequent events (Figure 6). Thus, it is possible that the weakened or dehisced central fovea might be a fairly subtle, early event, and focal proliferation of Mueller or glial cells along the vitreoretinal adherence produce the same appearance and could be indistinguishable from the appearance of vitreoretinal traction in a primary role despite the most accurate of imaging modalities.
Apparent contradictions of a vitreofoveal traction theory include the occurrence of full-thickness macular holes long after observing complete PVD,53 following scleral buckling procedure for rhegmatogenous detachments that presumably were due to complete PVD,54,55 and even well after vitrectomy for an unrelated diagnosis.56 In these eyes the cortical vitreous presumably was not available to mediate traction; perhaps a weakened, degenerated, inner retinal surface became attenuated independent of traction.
Regardless of the initiating mechanism, once the vitreous separates, an early (occult) macular hole could repair itself (especially if very small) via glial proliferation with little or no clinical symptoms or signs. If a full-thickness macular hole first presents as reopening of a previously undetected, self-healed macular hole, this could account for the predominance of (hyperplastic) Mueller cells and glial cells found in the removed opercula. If the discontinuity is too large, migration of reparative, proliferating glial cells is impeded,57 so a macular hole would enlarge as the glial cells which migrated around the hole edge onto the perifoveal internal limiting membrane progressively contract. The hydration theory is a novel concept that describes a mechanism by which a tiny dehiscence might permit increasing degrees of perifoveal cystic changes, and subsequently a dehiscence of a larger unit of inner retina (Figure 3).58
The OCT may have a role in identifying prognostic characteristics or in explaining postoperative results. Most patients with macular holes regain a gratifying amount of vision. The magnitude of improvement is variable, and may be disappointing in some. While patients with better preoperative visual acuity, shorter duration,59 earlier stage,60 and smaller macular hole size61,62 have better anatomic and visual success rates, these characteristics do not predict individual prognosis specifically. Other tests have also failed to offer a more specific prognosis, suggesting that a more complex interaction of factors is operative.63 Kusuhara, et al64 used an earlier model (OCT1), defined parameters, and attempted to identify a reproducible, accurate, and straightforwardly ascertainable prognostic algorithm, but without a substantial improvement in specificity. This was the first attempt to use the OCT as a prognostic tool.
Others have attempted to correlate or to explain postoperative vision with OCT characteristics. The OCT foveal morphology has been correlated to macular function in patients with closed macular holes with conflicting results.65,66 Kang positively correlated closure type (with or without neurosensory defect) and final visual outcome, but did not evaluate specific structural characteristics of the foveal region.65 Uemoto, using a similar approach, evaluated foveal contour (good shape and poor shape) and visual acuity in 86 eyes after successful macular hole surgery, but did not find a correlation when using a simple approach of grading the foveal shape.66
It would seem that an intact, layered neurosensory retina should correlate with visual acuity. However, foveal OCT findings in patients with anatomically closed holes frequently seem to contradict intuition — some patients with good visual acuity may have an asymmetric, odd-looking fovea, while others with poor visual acuity after successful macular hole surgery may present with a symmetric, near-normal foveal configuration. A study examining such eyes using OCT found, however, that certain specific structural features within the foveal architecture may be more important than others to restore visual function in patients with closed macular holes.67 Specifically, average photoreceptor thickness correlated with final visual acuity, confirming that physical integrity of the photoreceptor layer is most important for visual function. Furthermore, the integrity of the high reflective band representing the transition zone between inner and outer photoreceptor segments and the low reflective space between the transition zone and the RPE-choriocapillaris area, were found to be key features correlating with visual acuity. These may be useful indicators for follow-up management such as when considering cataract extraction in patients with previous macular hole surgery. Thus, the outer retina seems more important than the inner retina in terms of restoring optimal postoperative visual acuity, while the inner retina is likely more important to induce anatomic closure and clinical foveal morphology. However, clinical foveal morphology seems to be a relatively unimportant determinant of visual functions.
MANAGEMENT IMPLICATIONS
The therapeutic objectives in macular hole surgery involve one or more of the following: glial proliferation induction, relief of traction on the edges of the hole (surface or vitreoretinal), and providing a smooth template (gas bubble or hyaloid) for cell migration. While in selected cases surgery limited to surgical release of such a vitreofoveal adherence has been reported to be sufficient to induce closure of the macular hole,68 the usual case requires more relief of traction or stimulation of gliosis. Peeling of the ILM has become a commonly preferred step in contemporary macular hole surgical technique because it seems to improve closure rate, possibly by enhancing results.60-74 ILM peeling itself appears to be safe. Minor inner retinal damage has been demonstrated by transmission electron microscopy (TEM) in cadaver eyes undergoing ILM peeling.75 Clinically apparent effects were not seen despite the frequent, incidental finding of ILM fragments in TEM studies in the majority of removed epiretinal membrane (ERM) specimens.76 Poorer visual acuity was found when large ILM fragments were present in light micrographs of removed ERM specimens,77 as well as infrequent morphologic consequences. Subclinical microperimetry defects78 and subclinical paracentral scotomata scanning laser ophthalmoscopic (SLO) perimetry have also been reported.73 Iatrogenic punctate chorioretinopathy may occur due to repeated attempts at engaging an edge79 and self-limited subretinal hemorrhages80 have been reported with ILM removal.
Internal limiting membrane peeling is not an absolute requirement to induce macular hole closure,69,70 but generally increased anatomic and visual success rates reported as ILM peeling has been used more widely have led to its general acceptance.71,72,74,81,82 High success rates in lower prognosis macular hole cases, such as traumatic83 or highly myopic eyes,84 is compelling evidence of its efficacy.
Under the reasonable presumption that ILM removal is helpful, there has been much interest in perfecting its effective removal, including customized instrumentation85,86 and ILM staining to allow its visualization — most extensively studied using indocyanine green (ICG).87-96 Removing the ILM is unquestionably easier using ICG, but concerns regarding possible toxicity have been raised. ICG persists postoperatively for up to several months.97-103 It has been shown in vitro to be toxic due to external diffusion,104 and it induces a hyposmolality effect in cultured RPE cells.105 A host of other effects has been documented in vitro and in animal models suggesting a focus of toxicity on apoptosis of the RPE cells and through alteration of gene expression.106-112 ICG has been demonstrated to induce death of cultured glial cells at high concentrations,113 and in retinal ganglion cells.114 It has caused histological and ERG changes in rabbit eyes.115 Possible clinical examples of ICG toxicity have been reported as RPE atrophy,116,117 potentiation of phototoxicity,118 visual field defects,119 alteration of the ILM-retinal cleavage plane by allowing more inner retinal element adherence to the removed ILM,120 and optic neuropathy.121
Clinical reports have also suggested ICG toxicity. A clinical study showed no visual improvement in a series of 20 patients undergoing macular hole surgery using ICG.122 Histologic examination of removed epiretinal membrane specimens showed more cellular debris from eyes in which ICG was used compared to eyes without ICG, but more visual improvement was seen in ICG cases.123 Another report of 18 patients undergoing macular hole surgery found lower functional visual outcomes and more common visual field defects with ICG and questioned whether this was a toxic or mechanical effect.124 The hypothesis that light toxicity is potentiated by ICG is consistent with its spectral absorption, its effects on cultured RPE cells, cadaver eye studies, and the incidental finding of increased diode laser update in ICG stained eyes.125-129
Other studies, however, have not found evidence of the ICG toxicity. No effects were seen in an experimental study after exposing cultured RPE cells for 5 minutes, but after 10 minutes morphologic effects were found.130 Toxic effects were not seen in a clinical study of 18 eyes examined with SLO perimetry exams and photographs.131 A series of 37 eyes in which ICG was used showed a 97% anatomic success rate and 62% visual improvement rate including some to 20/20 indicating analogous results with standard series.132 Similar results were found in another study.88 These and other aspects defending the safety of ICG have been recently reviewed.133
Nevertheless, the conflicting opinions and results have caused many to advocate caution, further study, modifications in technique, or even restraint regarding the use of ICG.134,135 Sodium hyaluronate use has been suggested to block ingress of the ICG through the macular hole to the subretinal space.136 Alternate preparations of dye have been studied (eg, infracyanine green) without a definitive conclusion of a better toxicity profile.137-139 The technique of ICG staining has been described with a few permutations. Probably the most common concentration used has decreased from the 0.5%104,118-121 in initial reports to 0.1% or even 0.05%.122-124,131 However, less effective staining at lower concentrations may necessitate longer or repeated staining maneuvers.140,141 Shorter exposure times (15 seconds rather than 30 seconds to 3 minutes) have also been proposed as a means of minimizing toxicity risk.141,142 There is a general consensus to use as low of a concentration and time of exposure to the stain as possible. Some inject the dye without doing a temporary fluid-gas exchange, while others instill the dye into a small pool of residual fluid overlying the macula (as in the current study). A valid concern with the latter strategy is that partial fluid-air exchange effectively exposes the retina to a higher concentration, but yields a very faint staining which seemed to be at the visual threshold necessary to see an effect.
Other staining techniques utilizing trypan blue143-145 or triamcinolone acetonide146-150 may offer less toxicity while still facilitating reproducible and complete ILM removal. However, more studies are necessary to confirm pilot studies, especially regarding toxicities, as preliminary reports suggest possible toxicity with trypan blue,151-152 and induced intraocular pressure and cataracts are well-recognized potential complications of intraocular corticosteroids.153 Studies comparing ICG to trypan blue have generally shown a better toxicity profile for trypan blue.154-156
A reasonable clinical algorithm in the face of uncertainty regarding ICG use is to peel ILM without using ICG, as is usually possible, but if difficulty is anticipated or encountered, then ICG should be considered as a reasonable option. Cases ideal for ICG include those with compromised visibility or anatomic complexity.157
The element considered by most to be essential for success also presents the most formidable challenge to the patient — face-down positioning. Early investigators recommended up to 4-weeks positioning, but this has gradually been reduced to about 1 week without apparently compromising results. Some have suggested shorter intervals or even no positioning,158 but the success rates in some of those reports might not be as good as techniques using positioning, or may only be applicable in selected cases.159 Some patients are unable due to arthritis or dementia, or are unwilling to comply with a prone-positioning regimen. Complications have been reported with prolonged facedown positioning.160 In addition, air travel constraints may limit gas bubble use. These factors have forced surgeons to consider alternate treatment techniques.
Silicone oil tamponade has been evaluated as a solution to these constraints. An extra potential benefit of silicone oil would be more rapid recovery of postoperative vision. However, the need for re-operation to remove the oil must be considered, although it may be combined with cataract extraction in many patients. Initial results were encouraging,161,162 but later studies demonstrated anatomic results equivalent (at best) to using gas, but inferior visual results.163-165 Several reports have discussed possible retinal toxicity associated with silicone oil. Goldbaum discussed possible photoreceptor toxicity resulting from silicone oil exposure.161 Saitoh showed that 6-month silicone oil tamponade in a rabbit model may result in the accumulation of oil vacuoles within the optic nerve on electron microscopy.166 Similar accumulations may account for poor visual outcomes in macular hole patients.
Accordingly, gas tamponade remains the preferred surgical technique for patients undergoing macular hole repair, even if face-down positioning cannot be pursued. Shorter acting gas mixtures are preferable for patients desiring early air travel.
SUMMARY
Macular holes have gone from being an untreatable curiosity to become one of the most common and satisfying conditions for the vitreoretinal surgeon to treat. OCT imaging has offered markedly enhanced imaging of macular anatomy, allowing more accurate diagnosis and assessment of anatomic results, and it may offer some prognostic information. While pathogenesis is still incompletely understood, relief of vitreoretinal and tangential traction (whether causative or secondary) is a key objective of surgical repair. ILM peeling may not be necessary in all cases, but increases overall success rates without damaging retinal function, so it is pursued to achieve the traction relief objective. ICG seems safe, but because there are considerable questions about its possible toxicity it is probably best reserved for selected cases. While some relief in the postoperative face-down positioning regimen may be safe, silicone oil probably should be avoided as a tool to facilitate that goal.
Macular hole surgery, though highly successful by vitreoretinal surgical standards, may always be improved.
REFERENCES
1. Gass JDM. Idiopathic senile macular hole: Its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629-639.
2. Johnson RN, Gass JDM. Idiopathic macular holes: observations, stages of formation, and implications for surgical intervention. Ophthalmology. 1988;95:917-924.
3. Gass JDM. Reappraisal of biomicroscopic classification of stages
of develop
ment of a macular hole. Am J Ophthalmol. 1995;119:752-759.
4. Aaberg TM. Macular holes. A review. Surv Ophthalmol. 1970;15:139-162.
5. Zentmayer W. Hole at the macula. Ophthalmol Rec. 1909;18:198-200.
6. Aaberg TM, Blair CJ, Gass JDM. Macular Holes. Am J Ophthalmol. 1970;69:555-562.
7. Grignolo A. Fibrous components of the vitreous body. Arch Ophthalmol. 1952;47:760-774.
8. Kakahashi A, Schepens CL, Trempe CL. Vitreomacular observations:
II. Data on the pathogenesis of idiopathic macular breaks. Graefe's Arch Clin
Exp
Ophthalmol. 1996;234:425-433.
9. Frangieh GT, Green WR, Engel HM. A histopathologic study of macular cysts and holes. Retina. 1981;1:311-336.
10. Guyer DR, Green WR, de Bustros S, Fine SL. Histopathologic features of idiopathic macular holes and cysts. Ophthalmology. 1990;97:1045-1051.
11. Funata M. Wendel RT, de la Cruz Z, Green WR. Clinicopathologic study of bilateral macular holes treated with pars plana vitrectomy and gas tamponade. Retina. 1992;12:289-98.
12. Madreperla SA, Geiger GL, Funata M, de la Cruz Z, Green WR. Ophthalmology. 1994;101:682-686.
13. Rosa RH Jr., Glaser BM, de la Cruz Z, Green WR. Clinicopathologic correlation of an untreated macular hole and a macular hole treated by vitrectomy, transforming growth factor-beta2, and gas tamponade. Am J Ophthalmol. 1996;122:853-863.
14. Madreperla SA, McCuen BW II, Hickingbotham D, Green WR. Clinicopathologic correlation of surgically removed macular hole opercula. Am J Ophthalmol. 1995;120:197-207.
15. Ezra E, Munro PM, Chartens DG, et al. Macular hole opercula. Ultrastructural features and clinicopathologic correlation. Arch Ophthalmology. 1997;115:1381-1387.
16. Ezra E, Fariss RN, Possin DE, et al. Immunochemical characterization of macular hole opercula. Arch Ophthalmol. 2001;119:223-231.
17. Yoon HS, Brooks HL Jr, Capone A Jr, et al. Ultrastructural features of tissue removed during idiopathic macular hole surgery. Am J Ophthalmol. 1996;122:67-75.
18. Worst JGF. Cisternal systems of the fully developed vitreous body in the young adult. Tran Ophthalmol Soc UK. 1977;97:550-554.
19. Chu TG, Lopez PF, Cano MR, et al. Posterior vitreoschisis. An echographic finding in proliferative diabetic retinopathy. Ophthalmology. 1996;103:315-322.
20. Kakehashi A, Schepens CL, de Sousa-Neto A, et al. Biomicroscopic findings of posterior vitreoschisis. Ophthalmic Surg. 1993;24:846-850.
21. Kishi S, Koichi S. Posterior precortical vitreous pocket. Arch Ophthalmol. 1990;108:979-982.
22. Spaide R. Measurement of the posterior precortical vitreous pocket in fellow eyes with posterior vitreous detachment and macular holes. Retina. 2003;23:481-485.
23. Kishi S, Demaria C, Shimizu K. Vitreous cortex remnants at the fovea after spontaneous vitreous detachment. Int Ophthalmol. 1986;9:253-260.
24. Gass JDM. Muller cell cone, an overlooked part of the anatomy of the fovea centralis: hypothesis concerning its role in the pathogenesis of macular hole and foveomacular retinoschisis. Arch Ophthalmol. 1999;117:821-823.
25. Kishi S, Kamei Y, Shimizu K. Tractional elevation of Henle's fiber layer in idiopathic macular holes. Am J Ophthalmol. 1995;120:486-496.
26. Yamada E. Some structural features of the fovea centralis in the human retina. Arch Ophthal. 1969;82:151-159.
27. Gass JDM. Lamellar macular hole: a complication of cystoid macular edema after cataract extraction. Arch Ophthalmol. 1976;94:793-800.
28. McDonnell PJ, Fine SL, Hillis AI. Clinical features of idiopathic macular cysts and holes. Am J Ophthalmol. 1982;93:777-786.
29. Guyer DR, deBustros S, Diener-West M, Fine SL. Observations on patients with idiopathic macular holes and cysts. Arch Ophthalmol. 1992;110:1264-1268.
30. Folk JC, Boldt HC, Keenum DG. Foveal cysts: premacular hole condition associated with vitreous traction. Arch Ophthalmol. 1998;116:1177-1183.
31. Smiddy WE, Michels RG, Glaser BM, deBustros S. Vitrectomy for impending idiopathic macular holes. Am J Ophthalmol. 1988;105:371-376.
32. Jost BF, Hutton WL, Fuller DG, et al. Vitrectomy in eyes at risk for macular hole formation. Ophthalmology. 1990;97:843-847.
33. deBustros S. Vitrectomy for prevention of macular holes: Results of a randomized multicenter clinical trial. Ophthalmology. 1994;101:1055-1060.
34. Gass JDM, Joondeph BC. Observations concerning patients with suspected impending macular holes. Am J Ophthalmol. 1990;109:638-646.
35. Smiddy WE, Gass JDM. Masquerades of macular holes. Ophthalmic Surg. 1995;26:16-24.
36. Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular disease with optical coherence tomography. Ophthalmology. 1995;102:217-229.
37. Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of macular holes. Ophthalmology. 1995;102:748-756.
38. Azzolini C, Patelli F, Brancato R. Correlation between optical coherence tomography data and biomicroscopic interpretation of idiopathic macular hole. Am J Ophthalmol. 2001;132:348-355.
39. Chauhan DS, Antcliff RJ, Rai PA, Williamson TH, Marshall J. Papillofoveal traction in macular hole formation. Arch Ophthalmol. 2000;118:32-38.
40. Kishi S, Takahashi H. Three-dimensional observations of developing macular holes. Am J Ophthalmol. 2000;130:65-75.
41. Takahashi H, Kishi S. Tomographic features of a lamellar macular hole formation and a lamellar hole that progressed to a full-thickness macular hole. Am J Ophthalmol. 2000;130:677-679.
42. Haouchine B, Massin P, Gaudric A. Foveal pseudocyst as the first step in macular hole formation. Ophthalmology. 2001;108:15-22.
43. Uemura A, Uchino E, Doi N, Ohba N. Repair of impending macular hole in the early postoperative period as evaluated by optical coherence tomography. Arch Ophthalmol. 2002;120:398-400.
44. Menchini U, Virgili G, Giacomelli G, Cappelli S, Giansanti F. Mechanism of spontaneous closure of traumatic macular hole: OCT Study of one case. Retina. 2003;23:104-106.
45. Yamada H, Saka A, Yamada E, Nishimura T, Matsumura M. Spontaneous closure of traumatic macular hole. Am J Ophthalmol. 2002;134:340-347.
46. Mitamura Y, Saito W, Ishida M, Yamamoto S, Takeuchi S. Spontaneous closure of traumatic macular hole. Retina. 2001;21:385-389.
47. Parmar DN, Stanga PE, Reck AC, Vingerling JR, Sullivan P. Imaging of a traumatic macular hole with spontaneous closure. Retina. 1999;19:470-472.
48. Freund KB, Ciardella AP, Shah V, Yannuzzi LA, Fisher YA. Optical coherence tomography documentation of spontaneous macular hole closure without posterior vitreous detachment. Retina. 2002;22:506-509.
49. Tadayoni R, Massin P, Haouchine B, Cohen D, Erginay A, Gaudric A. Spontaneous resolution of small stage 3 and 4 full-thickness macular holes viewed by optical coherence tomography. Retina. 2001;21:186-189.
50. Imai M, Obshiro T, Gotoh T, et al. Spontaneous closure of Stage 2 macular hole observed with optical coherence tomography. Am J Ophthalmol. 2003;136:187-188.
51. Reese AB, Jones IR, Cooper WC. Macular changes secondary to vitreous traction. Am J Ophthalmol. 1967;64:544-549.
52. Reese AB, Jones IR, Cooper WC. Vitreomacular traction syndrome confirmed histologically. Am J Ophthalmol. 1970;69:975-977.
53. Gordon LW, Glaser BM, Ie, D, Thompson JT, Sjaarda RN. Full-thickness macular hole formation in eyes with a pre-existing complete posterior vitreous detachment. Ophthalmology. 1995;102:1702-1705.
54. Brown GC. Macular hole formation following rhegmatogenous retinal detachment repair. Arch Ophthalmol. 1998;106:765-766.
55. Smiddy WE. Atypical presentations of macular holes. Arch Ophthalmol. 1993;111:626-631.
56. Liphman WJ, Smiddy WE. Idiopathic macular hole following vitrectomy: implications for pathogenesis. Ophthalmic Surg Lasers. 1997;28:633-639.
57. Shubert HD, Kuang K, Kang F, Head MW, Fischbarg J. Macular holes: migratory gaps and vitreous as obstacles to glial closure. Graefe'sArch Clin Exp Ophthalmol. 1997;235:523-529.
58. Tornambe PE. Macular hole genesis: the hydration theory. Retina. 2003;23:421-424.
59. Wendel RT, Patel AC, Kelly NE, et al. Vitreous surgery for macular holes. Ophthalmology. 1993;100:1671-1676.
60. Ryan EH Jr, Gilbert HD. Results of surgical treatment of recent-onset full-thickness idiopathic macular hole. Arch Ophthalmol. 1994;112:1545-1553.
61. Ulrich S. Haritoglou C, Gass C, Schaumberger M, Ulbig MW, Kampik A. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002;86:390-393.
62. Ip MS, Baker BJ, Duker JS, et al. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol. 2002;120:29-35.
63. Tilanus MAD, Cuuypers MHM, Bemelmans NAM, et al. Predictive value of pattern VEP, pattern ERG and hole size in macular hole surgery. Graefe'sArch Clin Exp Ophthalmol. 1999;237:629-635.
64. Kusuhawa S, Escano MFT, Fujii S, et al. Prediction of postoperative visual outcome based on hole configuration by optical coherence tomography in eyes with idiopathic macular holes. Am J Ophthalmol. 2004;138:709-716.
65. Kang SW, Ahn K, Ham DI. Types of macular hole closure and their clinical implications. Br J Ophthalmol. 2003;87:1015-1019.
66. Uemoto R, Yamamoto S, Aoki T, Tsukahara I, Yamamoto T, Takeuchi S. Macular configuration determined by optical coherence tomography after idiopathic macular hole surgery with or without internal limiting membrane peeling. Br J Ophthalmol. 2002; 86:1240-1242.
67. Villate N, Lee JE, Venkatraman A, Smiddy WE. Photoreceptor layer features in eyes with closed macular holes: optical coherence tomography findings and correlation with visual outcomes. Am J Ophthalmol. 2005;139:280-289.
68. Spaide RF. Macular hole repair with minimal vitrectomy. Retina. 2002;22:183-186.
69. Smiddy WE, Feuer W, Cordahi G. Internal limiting membrane peeling in macular hole surgery. Ophthalmology. 2001;108:1471-1476.
70. Margherio RR, Margherio AR, Williams GA, Chow DR, Banach MJ. Effect of perifoveal tissue dissection in the management of acute idiopathic full-thickness macular holes. Arch Ophthalmol. 2000;118:495-498.
71. Park DW, Sipperley JO, Sneed SR, Dugel PU, Jacobsen J. Macular hole surgery with internal-limiting membrane peeling and intravitreous air. Ophthalmology. 1999;106:1392-1397.
72. Brooks HL Jr. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology. 2000;107:1939-1948.
73. Haritoglou C, Gass CA, Schaumberger M, Gandorfer A, Ulbig MW, Kampik A. Long-term follow-up after macular hole surgery with internal limiting membrane peeling. Am J Ophthalmol. 2002;134:661-666.
74. Mester V, Kuhn F. Internal limiting membrane removal in the management of full-thickness macular holes. Am J Ophthalmol. 2000;129:769-777.
75. Wolf S, Schnurbusch U, Wiedemann P, Grosche J, Reichenbach A, Wolburg H. Peeling of the basal membrane in the human retina. Ophthalmology. 2004;111:238-243.
76. Smiddy WE, Maguire AM, Green WR, et al. Idiopathic epiretinal membranes. Ultrastructural characteristics and clinicopathologic correlation. Ophthalmology. 1989;96:811-821.
77. Sivalingam A, Eagle RC Jr, Duker JS, et al. Visual prognosis correlated with the presence of internal-limiting membrane in histopathologic specimens obtained from epiretinal membrane surgery. Ophthalmology. 1990;97:1549-1552.
78. Haritoglou C, Gass CA, Schaumberger M, Ehrt O, Gandorfer A, Kampik A. Macular changes after peeling of the internal limiting membrane in macular hole surgery. Am J Ophthalmol. 2001;132:363-368.
79. Karacorlu M, Karacorlu S, Ozdemir H. Iatrogenic punctate chorioretinopathy after internal limiting membrane peeling. Am J Ophthalmol. 2003;135:178-182.
80. Nakata K, Ohji M, Ikuno Y, Kusaka S, Gomi F, Tano Y. Sub-retinal hemorrhage during internal limiting membrane peeling for a macular hole. Graefe's Arch Clin Exp Ophthalmol. 2003;241:582-584.
81. Al-Abdulla NA, Thompson JT, Sjaarda RN. Results of macular hole surgery with and without epiretinal dissection or internal limiting membrane removal. Ophthalmology. 2004;111:142-149.
82. Kimura T, Takahashi M, Takagi H, et al. Is removal of internal limiting membrane always necessary during stage 3 idiopathic macular hole surgery? Retina. 2005;25:54-58.
83. Kuhn F, Morris R, Mester V, Witherspoon CD. Internal limiting membrane removal for traumatic macular holes. Ophthalmic Surg Lasers. 2001;32:308-315.
84. Kwok AK, Lai TY. Internal limiting membrane removal in macular hole surgery for severely myopic eyes: a case-control study. Br J Ophthalmol. 2003;87:885-889.
85. Lewis JM, Park I, Ohji M, Saito Y, Tano Y. Diamond-dusted silicone cannula for epiretinal membrane separation during vitreous surgery. Am J Ophthalmol. 1997;124:552-554.
86. Rice TA. Internal limiting membrane removal in surgery for full-thickness macular holes. In Madreperla SA, McCuen BW (eds). Macular Hole: Pathogenesis, diagnosis, and treatment. Woburn, MA: Butterworth-Heinemann;1999:125-146.
87. Haritoglou C, Neubauer AS, Gandorfer A, Thiel M, Kampik A. Indocyanine green for successful repair of a long-standing macular hole. Am J Ophthalmol. 2003;136:389-391.
88. Sheidow TG, Blinder KJ, Holekamp N, et al. Outcome results in macular hole surgery: an evaluation of internal limiting membrane peeling with and without indocyanine green. Ophthalmology. 2003;110:1697-701.
89. Burk SE, Da Mata AP, Snyder ME, Rosa RH Jr, Foster RE. Indocyanine green-assisted peeling of the retinal internal limiting membrane. Ophthalmology. 2000;107:2010-2014.
90. Kadonosono K, Itoh N, Uchio E, Nakamura S, Ohno S. Staining of internal limiting membrane in macular hole surgery. Arch Ophthalmol. 2000;118:1116-1118.
91. Kwok AK, Li WW, Pang CP, et al. Indocyanine green staining and removal of internal limiting membrane in macular hole surgery: histology and outcome. Am J Ophthalmol. 2001;132:178-183.
92. Kwok AK, Lai TY, Yew DT, Li WW. Internal limiting membrane staining with various concentrations of indocyanine green dye under air in macular surgeries. J Ophthalmol. 2003;136:223-230.
93. Da Mata AP, Burk SE, Foster RE, et al. Long-term follow-up of indocyanine green-assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for idiopathic macular hole repair. Ophthalmology. 2004;111:2246-2253.
94. Gandorfer A, Messmer EM, Ulbig MW, Kampik A. Indocyanine green selectively stains the internal limiting membrane. Am J Ophthalmol. 2001;131:387-388.
95. Colucciello M. Delamination of the retinal internal limiting membrane using blunt dissection and indocyanine green staining. Retina. 2003;23:885-886.
96. Avci R, Avci B, Kaderli B, Cavusoglu I. A new surgical approach for indocyanine green-assisted internal limiting membrane peeling. Ophthalmic Surg Lasers. 2004;35:292-97.
97. Spaide RF. Persistent intraocular indocyanine green staining after macular hole surgery. Retina. 2002;22:637-639.
98. Horiguchi M, Nagata S, Yamamoto N, Kojima Y, Shimada Y. Kinetics of indocyanine green dye after intraocular surgeries using indocyanine green staining. Arch Ophthalmol. 2003;121:327-331.
99. Weinberger AW, Kirchhof B, Mazinani BE, Schrage NF. Persistent indocyanine green (ICG) fluorescence 6 weeks after intraocular ICG administration for macular hole surgery. Graefe's Arch Clin Exp Ophthalmol. 2001;239:388-90.
100. Machida S, Fujiwara T, Gotoh T, Hasegawa Y, Gotoh A, Tazawa Y. Observation of the ocular fundus by an infrared-sensitive video camera after vitreoretinal surgery assisted by indocyanine green. Retina. 2003;23:183-191.
101. Ciardella AP, Schiff W, Barile G, Vidne O, Sparrow J, Langton K, Chang S. Persistent indocyanine green fluorescence after vitrectomy for macular hole. Am J Ophthalmol. 2003;136:174-177.
102. Ashikari M, Ozeki H, Tomida K, Sakurai E, Tamai K, Ogura Y. Retention of dye after indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2003;136:172-174.
103. Nakamura H, Hayakawa K, Imaizumi A, Sakai M, Sawaguchi S. Persistence of retinal indocyanine green dye following vitreous surgery. Ophthalmic Surg Lasers. 2005;36:37-45.
104. Paques M, Genevois O, Regnier A, et al. Axon-tracing properties of indocyanine green. Arch Ophthalmol. 2003;121:367-370.
105. Stalmans P, Van Aken EH, Veckeneer M, Feron EJ, Stalmans I. Toxic effect of indocyanine green on retinal pigment epithelium related to osmotic effects of the solvent. Am J Ophthalmol. 2002;134:282-285.
106. Chang AA, Zhu M, Billson F. The internation of indocyanine green with human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2005;46:1463-1467.
107. Yam HF, Kwok AKH, Chan KP, et al. Effect of indocyanine green and illumination on gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:370-377.
108. Kawaji T, Hirata A, Inomata Y, Koga T, Tanihara H. Morphological damage in rabbit retina caused by subretinal injection of indocyanine green. Graefe's Arch Clin Exp Ophthalmol. 2004;242:158-164.
109. Rezai KA, Farrokh-Siar L, Ernest JT, van Seventer GA. Indocyanine green induces apoptosis in human retinal pigment epithelial cells. Am J Ophthalmol. 2004;137:931-933.
110. Enaida H, Sakamoto T, Hisatomi T, Goto Y, Ishibashi T. Morphological and functional damage of the retinal caused by intravitreous indocyanine green in rat eyes. Grafe's Arch Clin Exp Ophthalmol. 2002;240:209-213.
111. Maia M, Kellner L, DeJuan E Jr, et al. Effects of indocyanine green injection on the retinal surface and into the subretinal space in rabbits. Retina. 2004;24:80-91.
112. Mai M, Margalit E, Lakhanpal R, et al. Effects of intravitreal indocyanine green injection in rabbits. Retina. 2004;24:69-79.
113. Murata M, Shimizu S, Horiuchi S, Sato S. The effect of indocyanine green on cultured retinal glial cells. Retina. 2005;25:75-80.
114. Iriyama A, Uchida S, Yanagi Y, Tamaki Y, Inoue Y, et al. Effects of indocyanine green on retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:943-947.
115. Enaida H, Sakamoto T, Hisatomi T, Goto Y, Ishibashi T. Morphological and functional damage of the retina caused by intravitreous indocyanine green in rat eyes. Graefe's Arch Clin Exp Ophthalmol. 2002;240:209-213.
116. Hirata A, Inomata Y, Kawaji T, Tanihara H. Persistent subretinal indocyanine green induces retinal pigment epithelium atrophy. Am J Ophthalmol. 2003;36:353-355.
117. Maia M, Haller JA, Pieramici DJ, et al. Retinal pigment epithelial abnormalities after internal limiting membrane peeling guided by indocyanine green staining. Retina. 2004;24:157-160.
118. Engelbrecht NE, Freeman J, Sternberg P, et al. Retinal pigment epithelial changes after macular hole surgery with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2002;133:89-94.
119. Uemura A, Kanda S, Sakamoto Y, Kita H. Visual field defects after uneventful vitrectomy for epiretinal membrane with indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2003;136:252-257.
120. Gandorfer A, Haritoglou C, Gass CA, Ulbig MW, Kampik A. Indocyanine green-assisted peeling of the internal limiting membrane may cause retinal damage. Am J Ophthalmol. 2001;132:431-433.
121. Ando F, Yasui O, Hirose H, Ohba N. Optic nerve atrophy after vitrectomy with indocyanine green-assisted internal limiting membrane peeling in diffuse diabetic macular edema. Adverse effect of ICG-assisted ILM peeling. Graefe's Arch Clin Exp Ophthalmol. 2004;242:995-999.
122. Haritoglou C, Gandorfer A, Gass CA, Schaumberger M, Ulbig MW, Kampik A. Indocyanine green-assisted peeling of the internal limiting membrane in macular hole surgery affects visual outcome: a clinicopathologic correlation. Am J Ophthalmol. 2002;134:836-841.
123. Haritoglou C, Gandorfer A, Gass CA, Schaumberger M, Ulbig MW, Kampik A. The effect of indocyanine-green on functional outcome of macular pucker surgery. Am J Ophthalmol. 2003;135:328-337.
124. Gass CA, Haritoglou C, Schaumberger M, Kampik A. Functional outcome of macular hole surgery with and without indocyanine green-assisted peeling of the internal limiting membrane. Graefe's Arch Clin Exp Ophthalmol. 2003;241:716-720.
125. Blem RI, Huynh PD, Thall EH. Altered uptake of infrared diode laser by retina after intravitreal indocyanine green dye and internal limiting membrane peeling. Am J Ophthalmol. 2002;134:285-286.
126. Sippy BD, Engelbrecht NE, Hubbard GB, et al. Indocyanine green effect on cultured human retinal pigment epithelial cells: implication for macular hole surgery. Am J Ophthalmol. 2001;132:433-435.
127. Benz MS, Smiddy WE. Increased diode laser uptake in inner retinal layers after indocyanine green staining of the internal limiting membrane. Ophthalmic Surg Lasers. 2003;34:64-67.
128. Gandorfer A, Haritoslou C, Gandorfer A, Kampik A. Retinal damage from indocyanine green in experimental macular surgery. Invest Ophthalmol Vis Sci. 2003;44:316-323.
129. Haritoglou C, Priglinger S, Gandorfer A, Welge-Lussen U, Kampik A. Histology of the vitreoretinal interface after indocyanine green staining of the ILM, with illumination using a halogen and xenon light source. Invest Ophthalmol Vis Sci. 2005;46:1468-1472.
130. Ho JD, Tsai RJ, Chen SN, Chen HC. Cytotoxicity of indocyanine green on retinal pigment epithelium: implications for macular hole surgery. Arch Ophthalmol. 2003;121:1423-1429.
131. Weinberger AW, Schlossmacher B, Dahlke C, Hermel M, Kirchhof B, Schrage NF. Indocyanine-green-assisted internal limiting membrane peeling in macular hole surgery: a follow-up study. Graefe's Arch Clin Exp Ophthalmol. 2002;240:913-917.
132. Wolf S, Schnurbusch U, Wiedemann P, Grosche J, Reichenbach A, Wolburg H. Peeling of the basal membrane in the human retina. Ultrastructural effects. Ophthalmology. 2004;111:238-243.
133. DeMata AP, Riemann CD, Nehemy MB, et al. Indocyanine green-assisted internal limiting membrane peeling for macular holes to stain or not to stain? Retina. 2005;25:395-404.
134. Jackson TL. Indocyanine green accused. Br J Ophthalmology. 2005;89:395-396.
135. Kampik A, Sternberg P. Indocyanine green in vitreomacular surgery – (why) is it a problem? Am J Ophthalmol. 2003;136:527-529.
136. Cacciatori M, Azzolini M, Sborgia M, Coppola M, De Molfetta V. Sodium hyaluronate 2.3% prevents contact between indocyanine green and retinal pigment epithelium during vitrectomy for highly myopic macular hole retinal detachment. Retina. 2004;24:160-161.
137. Haritoglou C, Gandorfer A, Gass CA, Kampik A. Histology of the vitreoretinal interface after staining of the internal limiting membrane using glucose 5% diluted indocyanine and infracyanine green. Am J Ophthalmol. 2004;137:345-348.
138. Rivett K, Kruger L, Radloff S. Infracyanine-assisted internal limiting membrane peeling in the macular hole repair: does it make a difference? Graefe's Arch Clin Exp Ophthalmol. 2004;242:393-396.
139. Jackson TL, Vote B, Knight BC, El-Amir A, Stanford MR, Marshall J. Safety testing of infracyanine green using retinal pigment epithelium and glial cell cultures. Invest Ophthalmol Vis Sci. 2004;45:3697-3703.
140. Cheung BTO, Yuen CYF, Lam DSC, et al. Reply to ICG-assisted peeling of the retinal ILM. Ophthalmology. 2002;109:1039-1040.
141. Ando F, Sasano K, Suzuki F, Ohba N. Indocyanine green-assisted ILM peeling in macular hole surgery revisited. Am J Ophthalmol. 2004;135:886-887.
142. Narayanan R, Kennedy MC, Kamjoo S, et al. Toxicity of indocyanine green (ICG) in combination with light on retinal pigment epithelial cells and neurosensory retinal cells. Curr Eye Res. 2005;30:471-478.
143. Perrier M, Sebag M. Trypan blue-assisted peeling of the internal limiting membrane during macular hole surgery. Am J Ophthalmol. 2003;135:903-905.
144. Vote BJ, Russell MK, Joondeph BC. Trypan blue-assisted vitrectomy. Retina. 2004;24:736-738.
145. Teba FA. Mohr A, Eckardt C, et al. Trypan blue staining in vitreoretinal surgery. Ophthalmology. 2003;110:2409-2412.
146. Fraser EA, Cheema RA, Roberts MA. Triamcinolone acetonide-assisted peeling of retinal internal limiting membrane for macular surgery. Retina. 2003;23:883-884.
147. Horio N, Horiguchi M, Yamamoto N. Triamcinolone-assisted internal limiting membrane peeling during idiopathic macular hole surgery. Arch Ophthalmol. 2005;123:96-99.
148. Kimura H, Kuroda S, Nagata M. Triamcinolone acetonide-assisted peeling of the internal limiting membrane. Am J Ophthalmol. 2004;137:172-173.
149. Takasu I, Shiraga F, Otsuki H. Triamcinolone acetonide-assisted internal limiting membrane peeling in macular hole surgery. Retina. 2004;24:620-622.
150. Shah GK, Rosenblatt BJ, Smith M. Internal limiting membrane peeling using triamcinolone acetonide: histopathologic confirmation. Am J Ophthalmol. 2004;138:656-657.
151. Rezai KA, Farrokh-Siar L, Gasyna EM, Ernest JT. Trypan blue induces apoptosis in human retinal pigment epithelial cells. Am J Ophthalmol. 2004;138:492-495.
152. Narayanan R, Kenney MC, Kamjoo S, et al. Trypan blue: effect on retinal pigment epithelial and neurosensory retinal cells. Invest Ophthalmol Vis Sci. 2005;46:304-309.
153. Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intravitreous injection: a comprehensive review. Retina. 2004;24:676-698.
154. Gale JS, Proulx AA, Gonder JR, Mao AJ, Hutnik CML. Comparison of the in vitro toxicity of indocyanine green to that of trypan blue in human retinal pigment epithelium cell cultures. Am J Ophthalmol. 2004;138:64-69.
155. van Dooren BTH, Beekhuis H, Pels E. Biocompatibility of trypan blue with human corneal cells. Arch Ophthalmol. 2004;122:736-742.
156. Jackson TL, Hillenkamp J, Knight BC, et al. Safety testing of indocyanine green and trypan blue using retinal pigment epithelium and glial cell cultures. Invest Ophthalmol Vis Sci. 2004;45:2778-2785.
157. Kanda S, Uemura A, Sakamoto Y, Kita H. Vitrectomy with internal limiting membrane peeling for macular hole retinoschisis and retinal detachment without macular hole in highly myopic eyes. Am J Ophthalmol. 2003;136:177-180.
158. Tornambe PE, Poliner LS, Grote K. Macular hole surgery without face-down positioning. A pilot study. Retina. 1997;17:179-185.
159. Spaide RF. Closure of an outer lamellar macular hole by vitrectomy: hypothesis for one mechanism of macular hole formation. Retina. 2000;20:587-590.
160. Treister G, Wyganski T. Pressure sore in a patient who underwent repair of a retinal tear with gas injection. Arch Clin Exp Ophthalmol. 1996;234:657-658.
161. Goldbaum MH, McCuen BW, Hanneken AM, Burgess SK, Chen HH. Silicone oil tamponade to seal macular holes without position restrictions. Ophthalmology. 1998;105:2140-2147.
162. Karia N, Laidlaw A, West J, Ezra E, Gregor MZ. Macular hole surgery using silicone oil tamponade. Br J Ophthalmol. 2001; 85:1320-1323.
163. Lai JC, Stinnett SS, McCuen BW II. Comparison of silicone oil versus gas tamponade in the treatment of idiopathic full-thickness macular hole. Ophthalmology. 2003;110:1170-1174.
164. Voo I, Siegner SW, Small KW. Silicone oil tamponade to seal macular holes. Ophthalmology. 2001;108:1516-1517.
165. Couvillon S, Smiddy WE, Flynn HW. Jr, Eifrig CWG, Gregori. Outcomes of surgery for idiopathic macular hole: A case-control study comparing silicone oil with gas tamponade. Ophthalmic Surg Lasers. 2005;36:365-371.
166. Saitoh A, Taniguchi H, Gong H, et al. Long-term effect on optic nerve of silicone oil tamponade in rabbits: histological and EDXA findings. Eye. 2002;16:171-176.
William E. Smiddy, MD, is professor of ophthalmology specializing in macular holes, diabetic retinopathy, macular degeneration, macular disease, and vitreoretinal diseases and surgery at the Bascom Palmer Eye Institute in Miami, Fla. Dr. Smiddy has no financial interest in any of the information contained in this article. He can be e-mailed at wsmiddy@med.miami.edu.