PEER REVIEWED
Evolving Treatment Strategies for Diabetic Retinopathy
RON P. GALLEMORE, MD, PHD, DAVID S. BOYER, MD, EDGAR L. THOMAS, MD
The diabetic retinopathy studies of the 70s and 80s established the standards of care for managing patients with diabetic retinopathy. Focal laser, panretinal photocoagulation and vitrectomy surgery were all evaluated and found to be beneficial in select patients meeting specific diagnostic criteria. In addition, the risks and benefits of aspirin and tight control of blood glucose and blood pressure were also evaluated.
In recent years the indications for treatment have expanded along with the options available. Technological advances in surgical equipment have expanded the indications for vitrectomy, improving safety and outcomes. New applications of pharmacological agents, particularly steroids, have also been utilized to a greater extent. Even as frequency of use of some of these treatments increases, absent are the rigorous controlled randomized clinical trials for evaluating their safety and efficacy. Here we review how the standards of care for managing our diabetic patients in the 21st century are evolving.
|
|
|
|
Figure 1. Intravitreal injection of triamcinolone acetate. The eye is pretreated with topical antiobiotics and prepped with
Betadine. A lid speculum is inserted prior to the injection through the pars
plana. |
|
LASER TREATMENT FOR DIABETIC RETINOPATHY
Conventional argon laser (green, yellow or blue) has traditionally been used to treat clinically significant diabetic macular edema (DME). Recently, some surgeons have been utilizing diode laser as an alterative with low energy or subthreshold treatment. Pilot studies have now been published suggesting that lower energy diode laser may achieve the same improvement in edema with less scarring and subjective/objective field loss from the laser.1,2 Additional controlled studies will be required before more widespread use is observed.
Panretinal photocoagulation (PRP) for proliferative diabetic retinopathy may increase macular edema, reduce peripheral vision and be painful for the patient.3 We now utilize shorter duration pulses (e.g., 40 or 50 milliseconds instead of the conventional 100 milliseconds used in the Diabetic Retinopathy Study) of higher intensity to achieve comparable burn intensity with less patient discomfort. The need for retrobulbar blocks is essentially eliminated with this approach, and our clinical experience suggests comparable efficacy. The indirect laser appears to be comparable in efficacy to the contact lens for laser delivery and may provide improved patient comfort and ease of treatment, particularly for those who are unable to sit at the slit lamp.4 Our clinical impression is that far peripheral treatment may also reduce visual field loss and macular edema associated with conventional treatment because the indirect laser allows applications from the equator to ora serrata with relative ease.
The indirect argon and diode laser delivery systems can allow treatment of early cases of anterior hyaloidal fibrovascular proliferation. This uncommon condition usually occurs following primary vitrectomy for proliferative diabetic retinopathy.5 Early in the course of this potentially devastating condition, when blood behind the lens is present and recurrent vitreous hemorrhage has occurred but before extensive traction retinal detachment has developed, an air-fluid exchange may be used to clear the vitreous hemorrhage, and then indirect laser may be applied while viewing through the gas bubble to the region of the anterior vitreous base, ora serrata and pars plana where this anterior proliferation begins.
An alternative to laser treatment is cryotherapy to the areas of retinopathy. This is associated with more inflammation and discomfort but may be effective as well, particularly when visualization of the vitreous base and pars plana is obscured by media opacities. Revision vitrectomy is required for more advanced cases.6
STEROID INJECTIONS
Chronic, diffuse diabetic macular edema and a cystoid pattern of diabetic macular edema may respond poorly to laser treatment.7 In such cases, periocular and intravitreal steroid injections with triamcinolone acetate (Kenalog) have been utilized.8,9 The efficacy of intravitreal Kenalog (IVK) has yet to be studied in a large, prospective, randomized, controlled clinical trial, but there is growing evidence to support this strategy. In a recent study of 30 eyes of patients whose edema failed to respond to conventional laser treatment, IVK (0.4 mg in 0.1 cc) improved visual acuity from a mean of 0.17 to 0.36 at 3 months and reduced macular thickness measured with optical coherence tomography (OCT) from 476 um at baseline to 255 um at 3 months.10 A drop in vision and recurrent edema was observed and retreatment with IVK was performed in about 2/3 of eyes with an average of 6 months between injections. Preliminary results from another limited prospective clinical trial also demonstrated a reduction in macular thickness on OCT with IVK but did not demonstrate a statistically significant improvement in vision.11
IVK has also been found to be effective in some patients with proliferative diabetic retinopathy where neovascularization in the disc and elsewhere has been shown to regress.12 Diabetic papillopathy has also been reported to respond to IVK13 as have retinal vein occlusions for which diabetes mellitus is a risk factor.14
We utilize IVK just prior to cataract surgery in select patients to minimize recurrent leakage, such as those patients with a recent history of CSME, macular edema with vein occlusion or choroidal neovascularization. A recent prospective pilot study demonstrated that this approach appeard to be safe and effective in managing visual significant cataract in patients with clinically significant diabetic macular edema.15
While there is no standardized technique for IVK, (as indicated by the recent American Society of Retina Specialists Preferences and Trends Survey16, we advocate application of prophylactic antibiotics q.i.d. for 3 consecutive days prior to treatment or q5 minutes for 4 applications immediately prior to the injection if the former protocol is not practical.17 We also prep the lids and lashes with povidine iodine, place a drop of this solution into the inferior fornix and insert a lid speculum prior to injection (Figure 1). We use freshly opened triamcinolone acetate (40 mg/cc) in a 1 cc vial for single use. Complications associated with IVK include steroid-induced glaucoma (20 to 50%), secondary cataract (following multiple injections), sterile and infectious endophthalmitis (1 in 500 to 1 in 1000), vitreous hemorrhage (1 in 200) and retinal detachment (incidence unknown).7-19
|
|
|
|
|
|

|
|
|
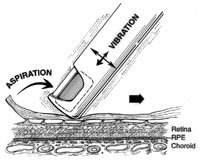
Figure 2. Indentation delamination of proliferative diabetic membranes. a. The cutter tip is positioned port-up near the membrane edge while the surface is gently indented by the instrument tip. b. Continuous aspiration produces traction on the membrane and the underlying fibrovascular pegs. c. Activation of the cutter while the membrane is under traction sheers the pegs from the underlying surface of the membrane while removing the membrane itself. Hemostasis is maintained by continual aspiration and cutting as the instrument is moved side to side, retreating from the membrane edge. |
VITRECTOMY
The diabetic retinopathy vitrectomy study (DRVS) demonstrated the efficacy of vitrectomy for severe proliferative diabetic retinopathy with nonclearing hemorrhage and progression despite laser treatment.20 At the time of the DRVS up to 20% of eyes progressed to no light perception. Today many advances have improved our success rates, including the advent of high-speed vitreous cutters, endolaser, wide-field viewing systems and pharmacological management of fibrin, retinal neovascularization and retinal edema.7-13,21,22
High-Speed Cutters
Several high-speed vitreous cutters are now available for diabetic vitrectomy and provide the advantage of more controlled vitreous removal and working on the retinal surface to remove complex diabetic membranes.23-26 (We utilize the Alcon InnoVit cutter for diabetic vitrectomy, although Bausch & Lomb and other companies have developed excellent cutters.) We have developed the technique of indentation delamination for removal of diabetic membranes and the concept of "one-step" diabetic vitrectomy (Figure 2). The high-speed cutters can be used to delaminate membranes from the retinal surface and, in the majority of cases, eliminate the need for forceps, scissors, endodiathermy, healon dissection of membranes and other maneuvers traditionally used to manage these complex patients.23
Once we became experienced with this approach, cautery and most other steps were usually eliminated from the case. With continuous aspirating as the membranes are delaminated, hemorrhage is continually removed and the membrane remains engaged. Once all the pegs are cut from the membrane (delamination is complete), they tend to autoconstrict and stop oozing. Transient elevation of the infusion pressure is used to control hemostasis as needed. The efflux control may be used to disperse any surface hemorrhage using the cutter tip itself rather than exchanging it for a soft tip. When combined with a wide-field viewing system, the entire retina can be visualized utilizing different magnifications, and a single lens can be used to complete the entire case. After vitrectomy is completed with the high-speed cutter, a curved-tipped endolaser probe can then be used to treat the entire peripheral retina. Scleral depression is then performed while viewing through the wide-field lens system to check the retinal periphery for tears.
No-Stitch Vitrectomy: 25-Gauge Systems, Beveled Incisions and Topical Anesthesia
Taking the hint from our anterior segment colleagues, vitreoretinal surgeons have been developing new and innovative ways to operate with less invasive techniques. Small gauge vitrectomy instrumentation is now available allowing no-stitch surgery.24-26 25-gauge vitrectomy instruments are now available from Bausch & Lomb, Alcon and others. These systems allow no-stitch transconjuntival insertion of vitreoretinal instrumentation, minimizing trauma to the eye and reducing the number of surgical steps. Each 25-gauge system utilizes a trocar-cannula system placed directly through the displaced conjunctiva as ports for instruments and infusion. The systems utilize a guillotine-action high-speed vitreous cutter with flow rates that can theoretically exceed conventional instrumentation. Surgeons may utilize a noncontact lens or sutureless contact lens ring to further minimize trauma.27 Advantages of 25-gauge vitrectomy include a quieter eye with minimal patient discomfort and potentially less postoperative inflammation with more rapid visual recovery. Disadvantages include practical limitations on flow for removal of dense hemorrhage and membranes when compared with standard 20-gauge high-speed cutters, bending of instrument shafts, and a limited choice of ancillary instrumentation. The required use of a transconjunctival trocar system is an easy, but uncommon, maneuver for most vitreoretinal surgeons and provides advantages of port localization, reduced vitreous incarceration, and quick, self-sealing sclerotomies.
While the 25-gauge systems are in development, other techniques are being used to allow a "no stitch" approach. Both beveled and wedge-shaped incisions have been utilized to create a water-tight seal with minimal vitreous incarceration.28,29 The conjunctiva can then be closed by appositional cautery or drying of the conjunctival flap over the sclerotomy site with a Weck-Cel sponge. While not ideal for complex cases requiring multiple instrument insertions, this no-stitch approach with conventional instrumentation works well for most cases.
This sutureless approach can be combined with topical and subconjunctival anesthesia alone in select cases. This eliminates the risks of retrobulbar anesthesia but requires a minimalist approach with manipulating the eye. More potent and fast-acting local anesthetics may be required before the no-stitch topical approach can be used more often in conventional vitreoretinal surgery.
Chemical Vitrectomy
Going beyond vitreous removal, the concept of chemical vitrectomy strives to dissolve or liquefy the vitreous in an office setting to facilitate or replace conventional vitreoretinal procedures. Purified ovine hyaluronidase (Vitrase) has now been evaluated in a randomized, controlled clinical trial for its effectiveness in managing nonclearing diabetic and nondiabetic vitreous hemorrhage.31 Liquification of vitreous would allow earlier treatment with panretinal photocoagulation and could potentially obviate the need for vitrectomy surgery in select patients. The Vitrase study did not achieve clinical significance based on visual acuity analysis, and Vitrase was not approved as a treatment for vitreous hemorrhage by the FDA. It was approved, however, as a dispersing agent for combination with injectable anesthetic agents for peri- and retro-bulbar injections. This raises the possibility of off-label use in vitreoretinal surgery once the agent is available.
Plasmin and chondroinase are similar agents that have been evaluated in limited clinical studies.32-36 In the meantime, some surgeons take advantage of the vitreous breakdown created by other maneuvers, including intravitreal injection of gas or steroids prior to surgery. Both of these maneuvers are known to promote breakdown of the vitreous gel and may promote the development of a posterior vitreous detachment (PVD), which can simplify diabetic vitrectomy. Induction of a PVD may also induce increased retinal traction and other complications. We do not advocate these approaches at the present time.
SILICONE IOL
While we still prefer gas over oil for our diabetic patients when a tamponade is required during vitrectomy surgery, in select cases oil may provide some advantages.30 Some evidence exists that oil can prohibit anterior segment neovascularization and may be best in patients with severe rubeosis, anterior hyaloidal fibrovascular proliferation and multiple recurrent vitreous hemorrhages following multiple vitrectomies for proliferative retinopathy. In complex cases with combined rhematogenous detachments, proliferative diabetic retinopathy and proliferative vitreoretinopathy, oil may be the best choice as well (Figure 3).
|
|
|
|
Figure 3. Management of complex combined
diabetic-rhegmatogenous retinal detachment. When a posterior break is present a scleral buckle may be avoided if all anterior traction has been removed. Reproliferation with redetachment may occur in such patients who may also have rubiosis or early neovascular glaucoma. While gas is used in most cases, if posterior PVR was present and there is
rubeosis, silicone oil may be preferable and the retina may be attached using the sandwich technique of flattening the posterior retina with perfluorocarbon and the anterior retina with a direct silicone oil-fluid exchange. |
|
IMAGING TECHNIQUES
Fluorescein angiography has been traditionally utilized to guide laser treatment in patients with diabetic macular edema and proliferative diabetic retinopathy. Based on the diabetic retinopathy studies, clinical exam dictates the need for treatment but angiography can guide the laser treatment and may even improve the standard of care.37
More recently, OCT has become a critical adjunct in the management of diabetic patients. When cystoid edema is identified on angiography and is resistant to laser treatment, OCT can be utilized to obtain quantitative measurements of retina thickness and the response to alternative therapies, including IVK and vitrectomy surgery. OCT can also help identify patients who may respond best to certain treatment options.
When marked cystoid edema is present, laser alone is often less effective and difficult to perform, and a steroid injection may provide greater benefit. If vitreous traction and/or a thickened preretinal membrane is identified on OCT, vitreous surgery for membrane removal and elimination of traction may be the treatment of choice.38 Some patients with cystoid diabetic edema may actually have subretinal fluid or a full thickness macular hole, both of which may not be detectable on clinical exam alone. Subretinal fluid may account for vision loss out of proportion to clinical findings and may portend a better prognosis than severe macular ischemia because the fluid may resolve with further treatment.
B-SCAN ULTRASONOGRAPHY
High resolution B-scan ultrasonography is now utilized to help plan surgical intervention and identify complications associated with diabetic retinopathy.39 Vitreous schisis is an important concept and ultrasonography can demonstrate this finding preoperatively.40,41 For optimal results the posterior hyaloid membrane must be removed since this is the scaffolding for new vessel growth and the cause of some cases of retina edema associated with vitreous traction.42 High resolution B-scans may reveal shallow macular tractional or exudative detachments that cannot be visualized clinically or on OCT (e.g., due to media opacity) and may precipitate earlier surgical intervention. Areas of posterior vitreous detachment (e.g., nasal vs temporal) may dictate the best location to initiate a vitrectomy. The presence of subtle areas of anterior vitreous traction may help account for the underlying cause of recurrent diabetic hemorrhage and support a decision for earlier revision vitrectomy rather than additional laser treatment alone.43
PHARMACOLOGICAL AGENTS
We now utilize IVK at the end of nearly all of our diabetic vitrectomy cases. Some surgeons also utilize dexamethasone added to the infusion fluid, which has now been shown to reduce postoperative inflammation in a randomized controlled study.44,45 In addition to reducing inflammation, the addition of a steroid during surgery may reduce retinal edema and reduce new vessel growth after vitrectomy surgery (e.g., anterior hyaloidal fibrovascular proliferation).
IVK is also used during vitreous surgery for visualization , allowing more complete removal of clear cortical vitreous.46,47 Surprisingly, a nonrandomized comparative study of 32 eyes in 30 patients did not demonstrate any benefit to the final outcomes of adding IVK in patients undergoing vitrectomy for diabetic retinopathy.48 This is not consistent with our clinical impression and this result may be due to sampling error in a small study and study design. A more rigorous study would be required to address the issue of utilization of IVK with vitreous surgery.
Low molecular weight heparin (e.g., enoxaparin sodium, [Lovenox]), 5-fluorouracil and tissue plasminogen activator have all been utilized to minimize fibrin formation during vitrectomy in complex diabetic cases.49 While a controlled study of these agents in diabetic eyes has yet to appear, these is compelling evidence for a potential benefit in select patients. We have select cases where marked early improvement was observed in patients with long standing retinal edema, but controlled studies will be required to assess the potential risks and benefits of this added step.
In patients with diabetic macular edema, or when a gas bubble injection is required during diabetic vitrectomy, intravenous acetazolamide (Diamox) may have benefits for the edema and secondary glaucoma, respectively. In select patients with diabetic macular edema, topical carbonic anhydrase inhibitors (e.g., dorzolamide) may reduce diabetic edema particularly when a component of cystoid macular edema is present. We use this on rare occasions where other options are either declined or not tolerated by the patient.
2004 PREFERENCES AND TRENDS SURVEY
The latest PAT Survey by the American Society of Retina Specialists, presented at the 2004 ASRS meeting in San Diego, shed light on the cutting edge treatments being utilized.16 The majority of respondents (84%) still wait to treat diabetic macular edema until it is clinically significant, although some initiate treatment before CSME develops. We do consider earlier laser if DME is present but CSME has not yet developed, such as in patients who are scheduled for or have recently had cataract surgery or PRP since both cataract surgery and PRP have been associated with an increased risk of progressive edema.
When CSME persists after maximal laser treatment has been applied, only 3% of respondentsreported that they would observe the patient; the majority would proceed with IVK (81%), and smaller percentages would recommend vitrectomy and posterior hyaloid peeling with (8%) or without (8%) ILM peeling.
Overall, 50% of specialists have performed 20 or more IVK injections for diabetic macular edema and many have observed the associated complications including intraocular inflammation (44%) and culture-positive endophthalmitis (12%).
SUMMARY
Advances in vitreoretinal surgery, pharmacology, laser technology and imaging techniques are changing the way we manage diabetic retinopathy. Some of the new treatment approaches, such as intravitreal steroid use, have been evaluated in uncontrolled studies and await evaluation in randomized clinical trials. In the meantime, it is our impression that these treatment approaches have improved outcomes and reduced complications for our diabetic retinopathy patients.
Address correspondence to: Ron P. Gallemore, MD, PhD, Retina-Vitreous Associates Medical Group, 1127 Wilshire Blvd., Suite 1620, Los Angeles, CA 90017. Phone: (213) 483-8810. Fax: (310) 318-5317. E-mail: retina2000@yahoo.com.
Dr. Gallemore, from the Retina-Vitreous Associates Medical Group, Los Angeles, Calif., and Jules Stein Eye Institute, UCLA School of Medicine, Los Angeles, Calif. Dr. Boyer, from the Retina-Vitreous Associates Medical Group and Doheny Eye Institute, USC School of Medicine, Los Angeles, Calif. Dr. Thomas, from Retina-Vitreous Associates Medical Group. Financial interest disclosure statements were not provided.
REFERENCES
1. Akduman L, Olk RJ. Subthreshold (invisible) modified grid diode laser photocoagulation in diffuse diabetic macular edema (DDME). Ophthalmic Surg Lasers. 1999 Nov-Dec;30(9):706-14.
2. Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004 Sept;88(9):1173-9.
3. Aroca PR, Salvat M, Fernandez J, Mendez I. Risk factors for diffuse and focal macular edema. J Diabetes Complications. 2004 Jul-Aug;18(4):211-5.
4. Gurelik G, Coney JM, Zakov ZN. Binocular indirect panretinal laser photocoagulation for the treatment of proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging. 2004 Mar-Apr;35(2):94-102.
5. Lewis H, Abrams GW, Foos RY. Clinicopathologic findings in anterior hyaloidal fibrovascular proliferation after diabetic vitrectomy. Am J Ophthalmol. 1987 Dec 15;104(6):614-8.
6. McCuen BW 2nd, Rinkoff JS. Silicone oil for progressive anterior ocular neovascularization after failed diabetic vitrectomy. Arch Ophthalmol. 1989 May;107(5):677-82. Erratum in: Arch Ophthalmol 1989 Jul;107(7):1030.
7. Guyot-Argenton C, El Maftouhi A. Failure of photocoagulation therapy for diabetic macular oedema. J Fr Ophtalmol. 2004 Jun;27(6 Pt 1):597-603.
8. Negi AK, Vernon SA, Lim CS, Owen-Armstrong K. Intravitreal triamcinolone improves vision in eyes with chronic diabetic macular edema refractory to laser photocoagulation. Eye. 2004 Sep 10.
9. Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003 Jan;121(1):57-61.
10. Ciardella AP, Klancnik J, Schiff W, Barile G, Langton K, Chang S. Intravitreal triamcinolone for the treatment of refractory diabetic macular oedema with hard exudates: an optical coherence tomography study. Br J Ophthalmol. 2004 Sep;88(9):1131-6.
11. Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R, Caulin C, Gaudric A. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004 Feb;111(2):218-24; discussion 224-5.
12. Karacorlu M, Ozdemir H, Karacorlu S, Alacali N. Regression of optic nerve head neovascularization in proliferative diabetic retinopathy after intravitreal triamcinolone. Regression of diabetic optic disc neovascularization after intravitreal triamcinolone. Int Ophthalmol. 2004 Mar;25(2):113-6.
13. Al-Haddad CE, Jurdi FA, Bashshur ZF. Intravitreal triamcinolone acetonide for the management of diabetic papillopathy. Am J Ophthalmol. 2004 Jun;137(6):1151-3.
14. Ip MS, Gottlieb JL, Kahana A, Scott IU, Altaweel MM, Blodi BA, Gangnon RE, Puliafito CA. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Arch Ophthalmol. 2004 Aug;122(8):1131-6.
15. Lam DS, Chan CK, Mohamed S, Lai TY, Lee VY, Lai WW, Fan DS, Chan WM. Phacoemulsification with intravitreal triamcinolone in patients with cataract and coexisting diabetic macular oedema: a 6-month prospective pilot study. Eye. 2004 Sep 24.
16. Pollack JS and Packo KH. 2004 American Society of Retina Specialists Preferences and Trends Survey.
17. Gallemore RP, Boyer DS (2004) Intravitreal kenalog injections. EyeNet, in press.
18. Nelson ML, Tennant MT, Sivalingam A, Regillo CD, Belmont JB, Martidis A. Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina. 2003 Oct;23(5):686-91.
19. Gillies MC, Simpson JM, Billson FA, Luo W, Penfold P, Chua W, Mitchell P, Zhu M, Hunyor AB. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004 Mar;122(3):336-40.
20. Two-year course of visual acuity in severe proliferative diabetic retinopathy with conventional management. Diabetic Retinopathy Vitrectomy Study (DRVS) report #1. Ophthalmol. 1985 Apr;92(4):492-502.
21. Taban M, Thomas EL, Boyer DS, Novack RL, Chu TG, Gallemore RP. Efficacy of verteporfin photodynamic therapy on laser-induced choroidal neovascularization and the ancillary effect on diabetic microvasculopathy. Curr Eye Res. 2004 Apr;28(4):291-5.
22. Sharma T, Gopal L. Recent developments in vitreoretinal surgery. J Indian Med Assoc. 2000 Dec;98(12):754-8, 760-2.
23. Gallemore RP, Thomas EL, Boyer DS et al. Indentation delamination of proliferative diabetic membranes utilizing a high speed vitreous cutter. Invest Ophthalmol Visual Sci. 2001;41(4): 3757.
24. Gallemore RP, Thomas EL, Boyer DS (2002) Minimally invasive vitreoretinal surgery. Review of Ophthalmology. 9(11): 48-62.
25. Hilton GF, Josephberg RG, Halperin LS, Madreperla SA, Brinton DA, Lee SS, Gordon SF.Office-based sutureless transconjunctival pars plana vitrectomy. Retina. 2002 Dec;22(6):725-32.
26. Fujii GY, De Juan E Jr, Humayun MS, Chang TS, Pieramici DJ, Barnes A, Kent D. Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery.Ophthalmol. 2002 Oct;109(10):1814-20.
27. Ikuno Y, Ohji M, Kusaka S, Gomi F, Nakata K, Futamura H, Tano Y. Sutureless contact lens ring system during vitrectomy. Am J Ophthalmol. 2002 Jun;133(6):847-8.
28. Van Kuijk FJ, Uwaydat S, Godley BF. Self-sealing sclerotomies in pars plana vitrectomy. Retina. 2001; 21:547-550.
29. Theelen T, Verbeek AM, Tilanus MA, van den Biesen PR. A novel technique for self-sealing, wedge-shaped pars plana sclerotomies and its features in ultrasound biomicroscopy and clinical outcome. Am J Ophthalmol. 2003;136(6):1085-92.
30. Gallemore RP, McCuen B II. (2000) Silicone oil tamponade in vitreoretinal surgery. In: S. Ryan ed., Retina, Chapter 127.
31. Boyer DS, Thomas EL, Novack RL. Intravitreal Injection of ACS-005 Hyaluronidase Produces Clearing of Vitreous Hemorrhage. Invest Ophthalmol Visual Sci. 1998; 39(4): 2345.
32. Williams JG, Trese MT, Williams GA, Hartzer MK. Autologous plasmin enzyme in the surgical management of diabetic retinopathy. Ophthalmol. 2001 Oct;108(10):1902-5; discussion 1905-6.
33. Asami T, Terasaki H, Kachi S, Nakamura M, Yamamura K, Nabeshima T, Miyake Y. Ultrastructure of internal limiting membrane removed during plasmin-assisted vitrectomy from eyes with diabetic macular edema.Ophthalmol. 2004 Feb;111(2):231-7.
34. Hesse L, Chofflet J, Kroll P. Tissue plasminogen activator as a biochemical adjuvant in vitrectomy for proliferative diabetic vitreoretinopathy. Ger J Ophthalmol. 1995 Nov;4(6):323-7.
35. Tanaka M, Qui H. Pharmacological vitrectomy. Semin Ophthalmol. 2000 Mar;15(1):51-61.
36. Sebag J. Shaken not stirred. Ophthalmol. 2001 Jul;108(7):1177-8.
37. Kylstra JA, Brown JC, Jaffe GJ, Cox TA, Gallemore R, Greven CM, Hall JG, Eifrig DE. The importance of fluorescein angiography in planning laser treatment of diabetic macular edema. Ophthalmol. 1999 Nov;106(11):2068-73.
38. Gallemore RP, Jumper JM, McCuen BW 2nd, Jaffe GJ, Postel EA, Toth CA. Diagnosis of vitreoretinal adhesions in macular disease with optical coherence tomography. Retina. 2000;20(2):115-20.
39. Rabinowitz R, Yagev R, Shoham A, Lifshitz T. Comparison between clinical and ultrasound findings in patients with vitreous hemorrhage. Eye. 2004 Mar;18(3):253-6.
40. Chu TG, Lopez PF, Cano MR, Freeman WR, Lean JS, Liggett PE, Thomas EL, Green RL. Posterior vitreoschisis. An echographic finding in proliferative diabetic retinopathy. Ophthalmol. 1996 Feb;103(2):315-22.
41. Schwatz SD, Alexander R, Hiscott P, Gregor ZJ. Recognition of vitreoschisis in proliferative diabetic retinopathy. A useful landmark in vitrectomy for diabetic traction retinal detachment. Ophthalmol.1996 Feb;103(2):323-8.
42. Capone A Jr, Panozzo G. Vitrectomy for refractory diabetic macular edema. Semin Ophthalmol. 2000 Jun;15(2):78-80.
43. Han DP, Lewandowski M, Mieler WF. Echographic diagnosis of anterior hyaloidal fibrovascular proliferation. Arch Ophthalmol. 1991 Jun;109(6):842-6.
44. Blankenship GW. Evaluation of a single intravitreal injection of dexamethasone phosphate in vitrectomy surgery for diabetic retinopathy complications. Graefes Arch Clin Exp Ophthalmol. 1991;229(1):62-5.
45. Chalam KV, Malkani S, Shah VA.Intravitreal dexamethasone effectively reduces postoperative inflammation after vitreoretinal surgery. Ophthalmic Surg Lasers Imaging. 2003 May-Jun;34(3):188-92.
46. Sonoda KH, Sakamoto T, Enaida H, Miyazaki M, Noda Y, Nakamura T, Ueno A, Yokoyama M, Kubota T, Ishibashi T. Residual vitreous cortex after surgical posterior vitreous separation visualized by intravitreous triamcinolone acetonide. Ophthalmol. 2004 Feb;111(2):226-30.
47. Sakamoto T, Miyazaki M, Hisatomi T, Nakamura T, Ueno A, Itaya K, Ishibashi T. Triamcinolone-assisted pars plana vitrectomy improves the surgical procedures and decreases the postoperative blood-ocular barrier breakdown. Graefes Arch Clin Exp Ophthalmol. 2002 Jun;240(6):423-9.
48. Jonas JB, Sofker A, Degenring R. Intravitreal triamcinolone acetonide as an additional tool in pars plana vitrectomy for proliferative diabetic retinopathy. Eur J Ophthalmol. 2003 Jun;13(5):468-73.
49. Asaria RH, Kon CH, Bunce C, Charteris DG, Wong D, Khaw PT, Aylward GW. Adjuvant 5-fluorouracil and heparin prevents proliferative vitreoretinopathy : Results from a randomized, double-blind, controlled clinical trial. Ophthalmol. 2001 Jul;108(7):1179-83.