PEER REVIEWED
Emerging Treatments for Diabetic Eye Disease:
Update on Clinical Trials
RAJEEV BUDDI, MD, DEAN ELIOTT, MD
The American Diabetes Association estimates that 18.2 million people in the United States (6.3% of the population) have diabetes. Diabetes is the leading cause of blindness among adults 20 to 74 years old, and diabetic retinopathy causes 12000 to 24000 new cases of blindness each year.1 Diabetic macular edema (DME; Figure 1) and the sequelae of proliferative diabetic retinopathy (PDR; Figure 2) are the main reasons for vision loss.
The current treatment strategy of blood glucose control, blood pressure control, timely application of laser photocoagulation, and pars plana vitrectomy where indicated has substantially reduced visual disability from diabetic retinopathy. These treatment modalities have proven beneficial in large, randomized, controlled, prospective clinical trials; however, laser treatment and vitrectomy have limitations and the potential for complications. One particularly challenging condition is DME, especially chronic diffuse DME. In patients with DME, laser treatment can slow but does not always halt the rate of vision loss, and laser rarely results in improved visual acuity.2 Vitrectomy surgery, with or without internal limiting membrane peeling, has also been attempted with limited success.
New pharmacologic therapies in advanced stages of development hold great promise in the treatment of diabetic retinal disease. We will review agents currently being evaluated in clinical trials and discuss the early results from these trials.
|
|
|
|
|
|

|
|
|
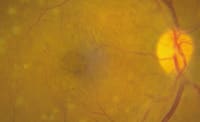
Figure 1 a. Fundus photograph demonstrates severe chronic cystoid macular edema in a patient with diabetic retinopathy. b. OCT of the same patient shows central foveal thickening at 772 µm. c. Late-phase FA demonstrates diffuse and cystoid macular edema. |
|
PHARMACOLOGIC THERAPIES CURRENTLY IN TRIALS
The characterization of molecular and cellular events causing retinal microvascular abnormalities in diabetic retinopathy has identified new targets for pharmacologic manipulation.3,4,5 Two of the molecules being targeted in current clinical trials are vascular endothelial growth factor (VEGF) and protein kinase C (PKC).
VEGF, a vascular endothelial cell mitogen and potent permeability factor, is produced by glial cells, retinal pigment epithelial cells, and vascular endothelial cells and is normally present in the retina and vitreous in low levels. Retinal hypoxia upregulates VEGF production, which results in abnormal angiogenesis and a marked increase in vascular permeability. The PKC family is a group of enzymes involved in signal transduction. The beta isoform has been shown to have an important role in regulating vascular permeability and is an important signaling component for VEGF.6,7 Chronic hyperglycemia of uncontrolled diabetes leads to increased cellular levels of diacylglycerol, which in turn activates PKC, especially the beta isoform. PKC beta increases the synthesis of VEGF and also contributes to the microvascular abnormalities in diabetic retinopathy. Inhibition of either VEGF or PKC beta moderates the microvascular complications seen in experimental animal models.8 Also, PKC beta inhibitors given orally may influence other diabetic complications such as renal insufficiency and peripheral neuropathy.9
New therapies currently under investigation that target the above molecules in diabetic retinopathy clinical trials include pegaptanib sodium (Macugen) and ruboxistaurin mesylate (LY333531). Pegaptanib sodium blocks the pathologic isoform VEGF 165, and ruboxistaurin is a selective PKC beta inhibitor. Another anti-VEGF agent, ranibizumab (Lucentis), the active fragment of a recombinant humanized antibody to VEGF, inhibits all isoforms of VEGF and is currently being evaluated only for age-related macular degeneration (AMD).10
In addition to these molecular therapies, intraocular corticosteroids are also being evaluated. In patients with DME, intravitreous injection of triamcinolone acetonide has been shown to be effective in improving visual acuity and reducing macular thickness measured by optical coherence tomography (OCT).11,12 This beneficial effect is transient and therefore requires repeated injections. The exact mechanism of action of corticosteroids in the treatment of DME is unknown. Corticosteroids decrease the breakdown of the blood-retinal barrier and are thought to downregulate the production of VEGF.13
The Intravitreous Steroid Injection Study (ISIS), a multicenter prospective trial, is investigating intravitreous triamcinolone acetonide for the treatment of macular edema secondary to diabetes and other etiologies. Several prospective randomized clinical trials are currently investigating sustained release devices for intraocular steroid delivery in the treatment of DME. These devices include an intravitreous fluocinolone acetonide implant (Retisert) and an intravitreous dexamethasone implant (Posurdex). An additional agent, anecortave acetate (Retaane), a steroid derivative with no corticosteroid activity, functions by inhibiting the proteolytic pathway necessary for the migration of endothelial cells into tissue stroma during angiogenesis and is currently being evaluated only for AMD.14
Another novel pharmacotherapy currently being evaluated is the intravitreous injection of a purified form of hyaluronidase (Vitrase) for the treatment of diabetic and other causes of vitreous hemorrhage (VH). At this time, the FDA has not approved the use of Vitrase for the treatment of VH.
Pegaptanib Sodium (Macugen)
Pegaptanib sodium is an aptamer (a synthetic oligonucleotide that binds to a target molecule) that selectively binds to the pathologic isoform of VEGF, VEGF165. The apatamer is pegylated (bound to polyethylene glycol) to increase its residence time in the eye, thus permitting intravitreous injections every 6 weeks. Results of a phase II randomized, placebo-controlled, double-masked, dose-finding, multicenter trial using pegaptanib in eyes with DME are now available.15
This study enrolled 169 patients who were otherwise eligible for thermal laser therapy for DME. Patients received varying doses (0.3 mg, 1 mg, and 3 mg) of pegaptanib via intravitreous injections or sham every 6 weeks for at least 12 weeks and then up to 30 weeks at the discretion of the investigators.
Preliminary data were statistically significant for the 0.3-mg dose of pegaptanib compared with control with respect to 0 or more line gainers (73% vs 51%, p = 0.02), 1 or more line gainers (59% vs 34%, p = 0.01) and 2 or more line gainers (34% vs 10%, p = 0.003) at 36 weeks. Preliminary data also showed a strong trend for pegaptanib-treated eyes to be more likely to gain 3 or more lines of vision than controls (18% vs 7%). OCT showed an even stronger trend toward a reduction in retinal thickness for pegaptanib (0.3 mg) compared with controls (50.79 µm vs 12.68 µm). In the geometric center of the macula, the odds of a decrease in retinal thickness of 75 µm or more was 4 times larger for the pegaptanib (0.3 mg) group vs controls (p = 0.0078). Preliminary safety data from this study suggest that pegaptanib appears to be well tolerated by patients with DME. In a prior study comprising approximately 1200 patients with AMD, there was a favorable safety profile for pegaptanib at all 3 doses.
Most adverse events in the study were mild, transient, and attributed by investigators to the injection procedure rather than the study drug. The injection procedure has risks, however, including endophthalmitis, retinal detachment, and intraocular hemorrhage. Endophthalmitis is a rare complication of intravitreous injection when careful attention is given to aseptic technique, including application of topical 5% betadine to the conjunctival surface and use of a sterile eyelid speculum.
Preliminary data suggest that inhibiting VEGF165 may be beneficial for patients with DME, and the lowest efficacious dose of pegaptanib sodium is 0.3 mg per injection. Like corticosteroid injections, this therapy requires repeated injections. Unlike corticosteroids, however, pegaptanib has a specific target. Currently, no long-term safety data are available for pegaptanib use for patients with DME.
|
|
|
|
|
|
|
Figure 2. a. Massive neovascularization and total traction retinal detachment in a patient with PDR. b. FA at 47 seconds shows severe capillary nonperfusion and extensive neovascularization. |
Ruboxistaurin Mesylate (LY333531)
Two clinical trials have evaluated the role of ruboxistaurin mesylate, an orally administered selective PKC beta inhibitor, in delaying or stopping the progression of diabetic retinopathy (PKC-DRS)16 and diabetic macular edema (PKC-DMES).17
The PKC-DRS was a multicenter, double-blind, placebo-controlled, parallel study that evaluated 252 patients with moderately severe to very severe nonproliferative diabetic retinopathy (ETDRS retinopathy severity grades 47B to 53E) in at least 1 eye. Eligibility criteria included visual acuity of 20/125 or better and no prior scatter photocoagulation. Patients were randomized to placebo or ruboxistaurin 8 mg, 16 mg, or 32 mg per day for 36 to 48 months. Eligibility and outcomes were assessed using stereoscopic fundus photographs taken at 6-month intervals. The primary outcome was a >= 3-step retinopathy progression on the ETDRS scale or application of scatter photocoagulation. Analysis was based on time to occurrence of the primary outcome using the intent-to-treat population.
At 42 months, Kaplan-Meier event rate estimates were 55%, 57%, 72%, and 52% in the placebo, 8-mg, 16-mg, and 32-mg groups, respectively (p = 0.54, 32 mg vs placebo). The 42-month rates for progression of >= 2 steps on the ETDRS scale in patients with nonproliferative diabetic retinopathy in both eyes were 72% and 49% for placebo and 32 mg, respectively (p = 0.048), a 32% risk reduction. Also, moderate visual loss rates (visual acuity loss of 15 letters) were lower in the 32-mg group than placebo at 12 months (12% vs 20%, p = 0.303), 24 months (8% vs 29%, p = 0.009), and 36 months (19% vs 28%, p = 0.346). The drug itself was well tolerated with no significant adverse events noted.
This study did not demonstrate a ruboxistaurin treatment effect on the primary endpoint of a >= 3-step retinopathy progression or application of scatter photocoagulation. However, ruboxistaurin 32 mg showed a potential beneficial effect in reducing moderate visual loss.
The PKC-DMES trial was a multicenter, double-masked, placebo-controlled trial that included 686 patients with DME that was not imminently sight threatening. Eligibility criteria included visual acuity of 20/32 or better and no prior photocoagulation. Patients were randomized to placebo or to riboxistaurin 4 mg, 16 mg, or 32 mg per day for >= 30 months. Eligibility and outcomes were assessed using stereoscopic fundus photographs taken at 3- to 6-month intervals. The primary outcome was progression of DME to involve or imminently threaten the center of the macula or application of focal/grid photocoagulation. Analysis was based on time to occurrence of the primary outcome using the intent-to-treat population.
At 36 months, Kaplan-Meier event rate estimates were 55%, 51%, 53%, and 47% in the placebo, 4-mg, 16-mg, and 32-mg groups, respectively (p = 0.23 overall, p = 0.15 for pair-wise comparison of 32 mg vs placebo). When subgroup analysis of these patients was conducted based on baseline HbA1c (HbA1c at baseline ¾10%, ¾75th percentile), placebo and ruboxistaurin (32 mg) event rates were 45% and 31%, respectively, a risk reduction of 31% (p = 0.019).
Treatment with ruboxistaurin did not prevent the primary endpoint of progression of DME to involve or imminently threaten the center of the macula or application of focal/grid photocoagulation. However, when patients with very poor glycemic control at enrollment (HbA1c >10%) were excluded from the analysis, ruboxistaurin 32 mg was associated with a reduction in DME progression.
Intravitreous Steroid Injection Study (ISIS)
ISIS is a prospective, multicenter, randomized, dose-escalation trial evaluating the utility of 2-mg and 4-mg doses of intravitreous triamcinolone acetonide injection (Kenalog-40) in eyes with macular edema secondary to diabetes, vein occlusion, pseudophakia, and retinal telangiectasia. The DME arm of the study enrolled 33 patients with persistent clinically significant macular edema 3 or more months after completion of what the treating physician considered maximal laser treatment, and ETDRS vision of ¾ 20/40. Patients with a history of IOP elevation >=30 mm Hg, elevated intraocular pressure in response to corticosteroids, or ocular surgery within the previous 3 months were excluded.
Six-month results (DME patients only) for improvement in visual acuity and total resolution of macular edema (by clinical examination) are now available.18 In the 2-mg group, a >=3 line visual improvement in 23% at 3 months and 0% at 6 months was noted, compared with 33% at 3 months and 21% at 6 months for the 4-mg group. In the 2-mg group, there was total resolution of macular edema in 20% at 3 months and 10% at 6 months, compared with 54% at 3 months and 25% at 6 months for the 4-mg group. These data indicate a trend toward greater efficacy and longer duration of effect with the 4-mg group when compared with the 2-mg group. During analysis, eyes were grouped as cystoid and noncystoid foveal edema based on a fluorescein angiogram (FA) graded in a masked fashion. Interestingly, 62% of eyes with cystoid foveal edema showed a >=3 line improvement in vision compared with only 9% for those with noncystoid foveal edema, a statistically significant difference. Intraocular pressure elevation of >=10 mm Hg was seen in 31% of patients, with a trend favoring the 4-mg group. A maximal IOP of >=30 mm Hg (range 30 to 36) was recorded in 28% of patients. None of these patients required glaucoma surgery.
This study demonstrates there is a potential for significant visual improvement following intravitreous triamcinolone injection in DME patients refractory to maximum laser treatment. This is a relatively cost-effective and technically easier in-office procedure compared with the corticosteroid implants discussed below. However, as shown in this study, visual acuity tends to regress over time as the macular edema recurs, and repeat injections are required. Also, the intraocular injection procedure has risks, as noted previously. IOP elevation was controlled medically in all patients. The study, however, did not have a control group.
The safety of intravitreous triamcinolone remains unresolved. The commercially available standard preparation (Kenalog-40) contains a preservative (benzyl alcohol) and an excipient (polysorbate-80), and is approved only for intramuscular use. Benzyl alcohol and polysorbate-80 have been implicated in postinjection sterile endophthalmitis. In addition to the standard preparation, a preservative-free formulation of triamcinolone is available from an independent compounding/pharmacy facility (New England Compounding Center).19 Another preservative-free triamcinolone acetonide preparation (Allergan) specifically formulated for use in the eye is currently being evaluated in clinical trials (SCORE: Standard Care vs Corticosteroid for Retinal Vein Occlusion Study; and the Diabetic Retinopathy Network Clinical Research Network Study: A Randomized Trial Comparing Intravitreal Tiamcinolone Acetonide and Laser Photocoagulation for Diabetic Macular Edema).
Infectious endophthalmitis is the most serious complication associated with intravitreous steroid injections. In a multicenter study involving 922 consecutive intravitreous triamcinolone injections, 87% of patients had culture-proven endophthalmitis in the first 6 weeks following the injection.20 Risk factors included diabetes mellitus, blepharitis, filtering blebs, and multiuse injection bottles. As previously stated, postinjection infective endophthalmitis can be minimized by scrupulous sterile technique, use of a lid speculum, and follow-up in the immediate postoperative period.
Fluocinolone Acetonide Implant (Retisert)
The 24-month results of a phase III study using the fluocinolone acetonide implant in patients with DME were recently released.21 The fluocinolone drug pellet is enclosed in a polymer, similar to the ganciclovir intravitreous implant (Vitrasert) used for cytomegalovirus retinitis. The 2 x 2 x 6-mm implant is inserted into the vitreous cavity through a pars plana incision and secured to the sclera with a suture. Fluocinolone is released at a constant rate over 3 years. The fluocinolone implant has the advantage of maintaining therapeutic levels in the target tissue (the macula) with minimal systemic exposure and an associated reduction in systemic side effects.
In this multicenter, randomized, masked, controlled trial (CDS FL-002), 80 patients with DME were randomized into an implant group (0.5 mg, n = 41; or 2 mg, n =11) and a standard-of-care group that received either macular grid laser or observation (SOC, n = 28). The 2-mg implant was discontinued early in the study because it showed no advantage over the lower dose. Inclusion criteria were a history of at least 1 macular laser procedure at least 3 months prior to enrollment, ETDRS visual acuity >= 20/400 and ¾ 20/50, and DME involving fixation and at least 1 disc area in size. Patients with a history of uncontrolled intraocular pressure or a history of ocular surgery within 3 months prior to enrollment were excluded. The primary endpoint evaluated from masked retinal photographs was resolution of retinal thickening at the center of the macula. Secondary endpoints were change in visual acuity from baseline, change in diabetic retinopathy score, and change in total area of hard exudates.
At 24 months, retinal edema at the center of the macula had resolved completely in 53.7% of the 0.5mg group compared with 28.6% of the SOC group (p = 0.039). In addition, 46.2% (0.5-mg group) showed a >2 grade improvement in retinal thickness at the center of the macula compared with 14.8% in the SOC group (p = 0.006). The diabetic retinopathy severity scores either remained stable or improved in 87.2% of the 0.5-mg implant group compared with 62.9% of the SOC group (p = 0.61). Mean change in visual acuity at 24 months was a gain of 9.3 ±14.4 letters for the 0.5-mg implant group and a loss of 1.9 ± 15.2 letters for the SOC group (p = 0.003).
Adverse events included cataract progression (77.4% in the 0.5-mg group vs 13.3% in the SOC group) and increased intraocular pressure (31.7% in the 0.5-mg vs 0.0% in the SOC group). Rates of cataract extraction were 74.2% in the 0.5-mg implant group and 13.3% in the SOC group. The majority of patients with elevated IOP were managed with drops, although 8 patients (19.5%) in the 0.5-mg implant group required trabeculectomy. Patient follow-up will continue at 3-month intervals for an additional 2 years.
The study found a statistically significant benefit in the primary endpoint of resolution of retinal thickening at the center of the macula and also an improvement in visual acuity and diabetic retinopathy score. This implant provides sustained levels of targeted fluocinolone and, as expected, is associated with a higher incidence of cataract and IOP elevation. It is possible to have multiple sequential implants in 1 eye. A larger phase III study (CDS FL-005) with similar inclusion criteria is currently underway and includes ~200 patients.
Dexamethasone Implant (Posurdex)
Posurdex, a biodegradable implant for extended release of dexamethasone, degrades in approximately 6 to 8 weeks. This cylindrical pellet is inserted into the region of the vitreous base through a small sclerostomy. Some safety and efficacy data (180 days) from a phase II randomized, multicenter, controlled trial using Posurdex for persistent macular edema has been presented.22
The study enrolled 306 patients with persistent macular edema, including DME (n = 172), retinal vein occlusion (n = 103), Irvine-Gass syndrome (n = 27), and uveitis (n = 14). Patients received either a single Posurdex implant containing 350 µg or 700 µg of dexamethasone, or no treatment (observation). Inclusion criteria were macular edema persisting at least 90 days following treatment (laser or medical management), visual acuity worse than 20/40 and attributable to macular edema, retinal thickening in the center of the fovea, and angiographic evidence of leakage involving the perifoveal capillary network. Patients with BCVA worse than 20/200, retinal neovascularization, or a history of pars plana vitrectomy or glaucoma were excluded. The primary efficacy endpoint was a 2-line or greater improvement in visual acuity. Secondary endpoints were change in retinal thickness measured by OCT, change in contrast sensitivity, and improvement in FA leakage as determined by masked grading.
Available results include all the etiological subgroups enrolled in the study. Ninety days after receiving the implant, a statistically significant primary efficacy outcome of a 2-line improvement in visual acuity was achieved with the 700-µg dose compared with the observation group, and this effect persisted at the 180-day evaluation. Also, at 180 days 19.4% of patients with the 700-µg implant showed an improvement of 3 or more lines compared with 7.9% in the observation group (p = 0.019). Patients treated with the 350-µg implant showed a trend toward improvement in visual acuity, indicating a dose response. Measures of edema correlated with improvement in visual acuity with a statistically significant decrease in retinal thickness and fluorescein leekage in both the 700-µg and 350-µg groups. Ninety days after implantation, contrast sensitivity was significantly better in the 700-µg group compared with the observation group. Ocular adverse events were more common in the implant groups and were mostly related to the pars plana insertion. These events included subconjunctival hemorrhage and vitreous hemorrhage, both of which were self limited. Patients did not show progression or onset of cataract; however, 17% of treated patients (700-µg group) showed a >=10 mm Hg IOP increase over baseline at some point during the study compared with 3% in the observation group. All IOP spikes were readily treated with antiglaucoma drops alone.
Although Posurdex has a relatively short duration of action (~35 days), the therapeutic effect lasts much longer, as seen in the phase II study where there was still effect in some eyes at 180 days. Sustained treatment benefit may require multiple implants. The drug-polymer combination technology used in Posurdex allows for the development of drug products that can deliver the drug to the targeted sites in the eye for up to 1 year. A single-use 22-gauge applicator preloaded with the implant is now available for office-based insertion of the implant through the pars plana, and this may reduce the adverse events associated with a conjunctival incision and sclerostomy. This applicator will be used in the ensuing phase III study.
Vitrase for Vitreous Hemorrhage Study (VVHS)
Vitrase is a highly purified preservative-free ovine hyaluronidase recently approved by the FDA for use as a spreading agent to facilitate the dispersion and absorption of other drugs. The VVHS, a randomized, double-masked, placebo-controlled study, was designed to evaluate the safety and efficacy of intravitreous injection (50 µL) of Vitrase for the treatment of VH.23
In this study, 1306 patients with severe VH for at least 1 month and a BCVA worse than 20/200 were randomized to 7.5 IU, 55 IU, or 75 IU of Vitrase or saline injection. The endpoints were a reduction in VH density sufficient to allow visualization of retinal details to facilitate diagnosis and the ability to perform laser treatment in at least 6 clock hours. At enrollment, 90% of patients had counting-finger vision or worse, and 76% were diabetic. Mean duration of VH was 120 days.
At each monthly visit, the proportion of Vitrase-treated diabetic patients with VH density reduction was statistically significant when compared with saline injections. At the 3-month evaluation, 43% (55 IU) and 39% (75 IU) of patients had VH density reduction compared with 28% of saline patients (p <0.001, p = 0.008). Proliferative diabetic retinopathy patients who received at least 6 clock hours of laser treatment by months 1, 2, and 3 were 11%, 23%, and 30% (55 IU) and 12%, 19%, and 26% (75 IU) compared with 5%, 13%, and 23% in the saline group.
Common adverse events (p ¾0.05) included iritis, hyperemia, irritation, pain, increased lacrimation, photophobia, photopsia, recurrent hemorrhage, and decrease in visual acuity. Most of these adverse events resolved within 1 month.
CONCLUSIONS
New pharmacologic interventions at the molecular level show great promise in treating visually disabling conditions such as DME and PDR. Early results from these clinical trials are extremely encouraging, and we eagerly await additional results and recommendations. The new modalities will most likely be used as adjuncts to the existing standard of care (laser photocoagulation) until they are compared with laser photocoagulation in clinical trials. In contrast to prior treatment strategies, most of these new modalities and techniques deliver the therapeutic agents directly to the ocular tissues. As a result, they require repeated intravitreous injections or sustained release devices, increasing the possibility of adverse events. At this time, an ideal delivery mechanism appears to be an injectable bioerodable extended delivery system. In the future, earlier intervention may be recommended in the prevention or treatment of diabetic retinal disease, and these new treatments will, it is hoped, decrease or eliminate the visual morbidity associated with this devastating disease. RP
Address correspondence to: Dean Eliott, MD, Retina Service, Kresge Eye Institute, Wayne State University School of Medicine, Detroit, Mich. Telephone: (313) 993-0871; Fax: (313) 577-2905; E-mail: deliott@med.wayne.edu.
From the Retina Service, Kresge Eye Institute, Wayne State University School of Medicine, Detroit, Mich. Dr. Eliott receives funding for clinical trials from Bausch & Lomb, Inc., Eyetech Pharmaceuticals, Inc., and Genentech, Inc. He receives travel support for periods of direct consultation from Alcon Laboratories, Inc. and Eyetech Pharmaceuticals, Inc. He has a financial interest in Control Delivery Systems, Inc.
REFERENCES
1. National Diabetes Fact Sheet, 2002. American Diabetes Association.
2. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. ETDRS Report Number 1. Arch Ophthalmol. 1985; 103 (12):1796-806.
3. Aiello LP, Cahill MT, Cavallerano JD. Growth factors and protein kinase C inhibitors as novel therapies for the medical management diabetic retinopathy. Eye. 2004; 18(2):117-25.
4. Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003; 26(9):2653-64.
5. Frank RN. Potential new medical therapies for diabetic retinopathy: protein kinase C inhibitors. Am J Ophthalmol. 2002; 133(5):693-8.
6. Nagpala PG, Malik AB, Vuong PT et al. Protein kinase C beta 1 overexpression augments phorbol ester-induced increase in endothelial permeability. J Cell Physiol. 1996;166(2):249-55.
7. Xia P, Aiello LP, Ishii H et al. Characterization of vascular endothelial growth factor's effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J Clin Invest. 1996; 98(9):2018-26.
8. Ishii H, Jirousek MR, Koya D, et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996; 3;272(5262):728-31.
9. Vinik A, Tesfaye S, Zhang D, et al. The MBBQ Study Group. LY333531 improves diabetic peripheral neuropathy (DPN) with symptoms. Presented at the 62nd Scientific Sessions of the American Diabetes Association, June 17, 2002.
10. Heier JS. Review of Lucentis (ranibizumab, rhuFab V2) phase I/II results: 6 month treatment of exudative AMD. IOVS. 2004; ARVO E Abstract 1109.
11. Martidis A, Duker JS, Greenberg PB et al. Intravitreous triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002; 109(5):920-7.
12. Bakri SJ, Kaiser PK. Intravitreous steroid injections for macular edema: way of the future? Retinal Physician. 2004; 1(1):40-45.
13. Wilson CA, Berkowitz BA, Sato Y, et al. Treatment with intravitreous steroid reduces blood-retinal barrier breakdown due to retinal photocoagulation. Arch Ophthalmol. 1992; 110(8):1155-9.
14. D'Amico DJ, Goldberg MF, Hudson H, et al. Anecortave Acetate Clinical Study Group. Anecortave acetate as monotherapy for treatment of subfoveal neovascularization in age-related macular degeneration: twelve-month clinical outcomes. Ophthalmology. 2003;110(12):2372-83.
15. Macugen(TM) (pegaptanib sodium injection) shows positive visual and anatomical outcomes in a phase 2 trial for patients with diabetic macular edema. Eyetech Pharmaceuticals press release, May 3, 2004. Available at: http://www.eyetk.com/investors /Press_Releases.asp.
16. Milton RC, Aiello LP, Davis MD, et al. Initial results of the protein kinase C beta inhibitor diabetic retinopathy study (PKC-DRS). Diabetologia. 2003; 46(suppl 2): A42.
17. Aiello LP, Davis MD, Milton RC, et al. Initial results of the protein kinase C beta inhibitor diabetic macular edema study (PKC-DMES). Diabetologia. 2003; 46(suppl 2): A42.
18. Pollack JS. Intravitreous steroid injection studies: diabetic macular edema. Presented at the 22nd Retina Society meeting, August 16-20, 2004.
19. Bakri SJ, Shah A, Falk NS, Beer PM. Intravitreal preservative-free triamcinolone acetonide for the treatment of macular oedema. Eye. 2004. Advance online publication.
20. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136(5):791-6.
21. Pearson P, Baker C, Eliott D, et al. Fluocinolone acetonide intravitreous implant for diabetic macular edema: 2 year results. Presented at the annual Association for Research in Vision and Ophthalmology meeting, April 25-29 2004.
22. Haller JA. The steroid device: the Oculex study. Presented at the Retina Subspecialty Day, American Academy of Ophthalmology meeting, November 15-18, 2003.
23. Kuppermann BD, Thomas EL, Grillone LR. Vitrase reduces vitreous hemorrhage density facilitating earlier laser therapy in patients with PDR. The Vitrase Study Group. Presented at the Retina Society meeting, September 18-21, 2003.