Reaffirming the Gold Standard
Even with the development of new imaging technology, fluorescein angiography remains a valuable tool for diagnosing and treating AMD.
BY MITHLESH SHARMA, MD AND LAWRENCE J. SINGERMAN, MD, FACS
Fluorescein angiography (FA) is an important diagnostic test for exudative age-related macular degeneration (AMD). Although many physicians use indocyanine green (ICG) angiography as a complementary test, major clinical trials still use FA to visualize choroidal neovascularization (CNV) in patients with AMD. In this article, we discuss why FA remains the gold standard for identifying CNV and monitoring disease progression in patients with AMD.
|
|
Identifying Recurrent CNV |
|
|
Patients who receive laser treatment for CNV often have persistent or recurrent leakage, even after apparently successful photocoagulation therapy. In the Macular Photocoagulation Study (MPS), more than 50% of eyes had persistent or recurrent leakage 6 weeks after receiving laser treatment for extrafoveal or juxtafoveal lesions. The MPS also found that these patients had an increased risk of severe vision loss compared with patients who did not develop persistent or recurrent CNV. All patients should be checked for recurrent CNV 3 to 4 weeks after their initial laser treatment. At this time, slit-lamp biomicroscopy and fluorescein angiography can detect CNVs before they become too large for treatment. |
|
|
FINDING CNV
Because patients age 65 or older with known dry AMD have a high risk of developing wet AMD with CNV, routine follow-up should include screening for new onset metamorphopsia or scotomas. Specifically, watch for:
► Localized retinal or pigment epithelial elevations caused by underlying fluid, blood or fibrovascular tissue
► Intra- or subretinal lipid deposits
► Cystic retinal edema overlying CNV.
Occasionally, these fundus lesions are subtle and difficult to detect, even with a slit-lamp biomicroscope and a macular contact lens. Under these conditions, FA is necessary, not only to confirm the presence of CNV but also to ascertain the location, type and dimensions of retinal lesions.
Fluorescein angiography also is useful for detecting recurrent or persistent CNV after thermal laser or photodynamic therapy (PDT).
|
|

|
|
|
Fig. 1: Illustration of extrafoveal, juxtafoveal and subfoveal CNV. FAZ--foveal avascular zone. |
In one study, FA identified questionable or definitive recurrent CNV in 12% of eyes that appeared CNV-free under slit-lamp biomicroscopy.1 The sooner we can find and treat CNV, the more likely we can limit hemorrhagic, exudative and cicatrical complications that cause vision loss.
Before we can begin therapy, we need to determine the type of lesion we'll be treating.
CHARACTERIZING LESIONS
As illustrated in Figure 1, CNV commonly is categorized into these three major types, based on their anatomic relationship to the fovea:
► Extrafoveal CNV is found between 200 µm and 2500 µm from the geometric center of the foveal avascular zone.2
► Juxtafoveal CNV is confined to an area up to 199 µm from the geometric center of the foveal avascular zone. This area may include portions of the foveal avascular zone.
► Subfoveal CNV is directly beneath the geometric center of the foveal avascular zone.
|
The Many Faces of AMD |
|||||
|
Beside CNV, AMD can manifest as window defects secondary to geographic atrophy (See Figure 2) and staining of the drusen in dry AMD, and serous pigment epithelium detachment (PED) in wet AMD. Fluorescein angiography shows PED as an intense, early-frame hyperfluorescence that stays the same size while increasing in intensity in late angiography frames. FA may or may not demonstrate the presence of CNV associated with serous PED. Treatment strategies for serous PED remain controversial. All the major AMD treatment trials, including the Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) study and the Verteporfin in Photodynamic Therapy (VIP) trial, excluded eyes with serous PED.
|
|
||||
|
|
CNV also is classified according to leakage patterns. The Macular Photocoagulation Study (MPS) identified two basic lesion patterns on fluorescein angiogram: Classic and occult.
Classic CNV has well-delineated boundaries in early angiogram frames.
These lesions are further divided into predominantly classic, in which the classic component occupies more than 50% of the entire lesion at baseline, and minimally classic, in which the classic component occupies between 0% and 50% of the entire lesion area. In later frames, FA shows CNV as progressive dye leakage that causes pooling in the overlying subsensory retinal space.
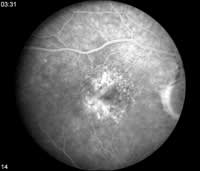
Occult CNV angiographic patterns don't conform to those seen with classic CNV (See Figure 3).
Fluorescein angiograms of occult CNV include fibrovascular pigment epithelium detachment (PED) and lesions that show leakage of undetermined source in late frames.
These angiographic distinctions are important for determining which patients with subfoveal CNV are most likely to benefit from photodynamic therapy (PDT). In the MPS, only patients with classic CNV were considered appropriate thermal laser candidates.
GUIDING TREATMENT
Fluorescein angiography plays a dual role in treating AMD by helping us identify the size and type of CNV lesions and by setting treatment guidelines. Some types of therapy are better suited for treating certain CNV.

|

|
| Fig. 2A: Right eye with geographic atrophy and extrafoveal CNV. Filling of classic CNV inferotemporal to the fovea in the venous angiogram phase. | Fig. 2B: Left eye with geographic atrophy between the disc and the fovea. |
► Photocoagulation therapy. This treatment, which uses a heat-generating laser to seal leaky retinal blood vessels, is most commonly used to treat extrafoveal CNVs. Most laser applications don't interfere with visual acuity because they're focused on lesions outside the macular center. However, photocoagulation will cause a scotoma adjacent to fixation wherever an intense thermal laser burn is placed.
Following MPS results, some physicians use photocoagulation to treat juxtafoveal and subfoveal lesions, but the advent of PDT has rendered this approach nearly obsolete. Common risks associated with photocoagulation therapy for these lesions include foveal ablation and immediate vision loss, so only eyes with very small lesions may benefit from this therapy. However, some evidence suggests that these eyes often do better with PDT than do eyes with larger lesions.3,4
|
|
|
|
|
Fig. 3: Late-phase fluorescein angiograms of occult CNV show leakage of undetermined source. |
► Photodynamic therapy (PDT). Over the past few years, PDT has become the preferred treatment for juxtafoveal and subfoveal CNVs. An increasing number of retina specialists are using PDT to treat juxtafoveal CNV, particularly in cases where hemorrhage obscures the foveal side of the CNV complex's posterior boundary. Data from the 1-year mark of the Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) study showed that 67% of eyes that received PDT for predominantly classic CNV lost fewer than 15 letters on the ETDRS chart, compared with 39% of untreated eyes. Typically, successful PDT treatment for predominantly classic CNV requires four to six treatments over 2 years and includes FA reevaluation every 3 months after each treatment. In general, patients with subfoveal CNV should receive PDT unless they develop severe vision loss immediately after PDT or if they're allergic to verteporfin.
PDT may even be appropriate for some patients with minimally classic CNV, particularly those with a documented classic component of CNV that's increasing and approaching 50% of the entire lesion. Data from the TAP study and the Verteporfin in Photo-dynamic Therapy (VIP) trial suggest a relationship between treatment efficacy and lesion size in eyes with minimally classic lesions or occult lesions without classic components. In these subgroups, eyes with smaller lesions not exceeding 4 disc areas had visual acuity outcomes similar to those with predominantly classic lesions. Research-ers are investigating the benefits of treating minimally classic lesions with PDT in a prospective, multicenter study.
Despite efficacy in treating other types of lesions, PDT appears to be less effective for treating entirely occult CNV. Evidence from the AMD arm of the VIP trials showed that of patients with occult CNV, those with small lesions (< 4 MPS disc areas) or poorer visual acuities (20/50 or worse) are more likely to benefit from PDT.

|

|

|
|
Fig. 4A: Right eye with large patches of geographic atrophy surrounding the fovea. |
Fig. 4B: Fluorescein angiography demonstrates uniform hyperfluorescence without leakage in the areas of perifoveal geographic atrophy. Note the brightly hyperfluorescent area of CNV without leakage inferonasal to the fovea that could easily be missed on clinical examination without FA. | Fig. 4C: Regression of leakage in the right eye after thermal laser treatment to the CNV. |
ENDURING INFLUENCE
Over the years, FA has significantly contributed to the diagnosis and treatment of exudative AMD. This reliable imaging technique lets us not only monitor disease progression, but also evaluate the efficacy of new AMD therapies.
Newer, more advanced imaging modalities, such as ICG testing with the Heidelberg Retina Angiograph 2 and studies with the StratusOCT, may come along, but we believe that FA will continue to play an important role in managing CNV.
|
Subfoveal Occult Lesions and PDT |
|
In April 2004, the Centers for Medicare & Medicaid Services (CMS) determined that a 2-year follow-up to the Verteporfin in Photodynamic Therapy (VIP) trial provided sufficient evidence to support using PDT to treat subfoveal occult lesions without classic CNV and subfoveal minimally classic CNV. PDT therapy is considered reasonable and necessary when: 1. Lesions are smaller than the equivalent of four discc areas at the time of initial treatment or within the 3 months before initial treatment. 2. Lesions show evidence of progression resulting in decreased visual acuity by a minimum of 5 standard chart letters, increased size by one or more disc areas or the appearance of blood associated with the lesion. |

|
Dr. Sharma is a retina specialist at Retinal Associates of Cleveland. |

|
Dr. Singerman is a clinical professor of ophthalmology at Case Western Reserve University, University Hospitals of Cleveland and he is president of Retina Associates of Cleveland. |
REFERENCES
1. Sykes SO, Bressler NM, Maguire MG, et al. Detecting recurrent choroidal neovascularization. Comparison of clinical examination with and without fluorescein angiography. Arch Ophthalmol. 1994;112:1561-1566.
2. Singerman LJ, Hionis R. In discussion: Vander J, Morgan C, Schatz H. Growth rate of subretinal neovascularization in age-related macular degeneration. Ophthalmology. 1989;96:1422-1426, discussion 1426-1429.
3. Blinder KJ, Bradley S, Bressler NM, et al. Effect of lesion size, visual acuity, and lesion composition on visual acuity change with and without verteporfin therapy for choroidal neovascularization secondary to age-related macular degeneration: TAP and VIP report no. 1. Am J Ophthalmol. 2003;136:407-418.
4. Bressler NM. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization-Verteporfin In Photodynamic Therapy Report 2. Am J Ophthalmol. 2002;133:168-169.









