Anecortave Acetate: Treatment for Age-Related Macular Degeneration
ALLEN C. HO, MD
Age-related macular degeneration (AMD) is the leading cause of severe, permanent vision loss in the industrialized world.1 Although the nonexudative (dry) form is more common, the exudative (wet) form accounts for the majority of patients with severe central vision loss. The etiology underlying AMD is less well understood than the biology of angiogenesis. AMD prevalence increases with advancing age. A recent study of Medicare patients 65 years old and older found that the prevalence of AMD increased from 5% to 27% over an 8-year period, and the exudative form increased from 0.5% to 5.2%.2 The United States population >64 years of age is projected to double by 2025; therefore, it is imperative to identify therapeutic interventions that can halt or delay the progression of visual loss in older Americans.
Until 2000 only laser photocoagulation was approved for treating choroidal neovascularization (CNV) associated with exudative AMD. However, it was found that laser photocoagulation sometimes worsened the condition, causing immediate and permanent loss of central vision. Then in 2000 photodynamic therapy (PDT) with verteporfin (Visudyne) was approved for treating selected subfoveal AMD lesions. While verteporfin delays vision loss, typically it does not stop the loss in the majority of patients.3 Additional interventions are now being explored, including transpupillary thermotherapy, radiation therapy, surgical intervention, and angiostatic pharmacologic agents.
|

|
|
|
Figure 1. Chemical structure of anecortave acetate 15 mg for depot suspension. Source: Alcon Laboratories, Inc. |
|
ANECORTAVE ACETATE AND MECHANISM OF ANTIANGIOGENESIS
In 1985 it was discovered that certain drugs with steroidal structures inhibited neovascularization in the chick embryo model, and these steroid-like compounds were devoid of conventional steroid hormone activities.4 Furthermore, they required heparin as a cofactor for angiostatic activity. Additional research led to the development of anecortave acetate, an antiangiogenic synthetic analog of cortisol that does not require heparin as a cofactor.5 Anecortave acetate is currently being investigated in clinical trials as a treatment option for AMD.
Structurally, anecortave acetate differs from cortisol by the removal of 11-beta hydroxyl, addition of a double bond at the C9-11 position, and addition of an acetate group at the C21 position, which results in a novel angiostatic cortisene that enhances angiostatic activity but does not exhibit typical glucocorticoid-receptor-mediated activity (Figure 1). These modifications eliminated most antiinflammatory activity typical of the initial cortisol molecule. Preclinical studies confirmed that the molecule lacks the typical immunosuppressant activity normally associated with steroidal structures. The drug does not inhibit IL-1beta secretion in U937 human lymphoma cells pretreated with lipopolysaccharide,6 nor does the novel cortisene exhibit antiinflammatory activities in the rat and rabbit models of endotoxin uveitis,7 or the carrageenan-induced rat foot paw edema model of inflammation. Despite an apparent lack of glucocorticoid activity, anecortave acetate possesses a broad range of antiangiogenic properties in numerous animal species and tissues independent of the initial angiogenic stimulus applied. Thus, the drug's therapeutic value for treating a variety of neovascular diseases appears quite promising.
Mechanism of Antiangiogenesis
Although neovascularization is a complex process, it can be broken down into several stages.8 The growth of new blood vessels begins with an angiogenic signal. Conditions such as ischemia or inflammation can induce a variety of angiogenic factors, including vascular endothelial growth factor (VEGF) or basic fibroblast growth factor (bFGF), which in turn induce vascular endothelial cell (VEC) proliferation. VEGF also increases vascular permeability. In response to an angiogenic signal, VECs produce extracellular proteases such as urokinase-type plasminogen activator (uPA) and various matrix metalloproteinases, which degrade the basement membrane surrounding VECs. The VECs can now leave the established vasculature to proliferate and migrate through interstitial tissues toward the angiogenic signal. In the last phases of angiogenesis, a capillary lumen forms a patent capillary that permits blood flow, and a basement membrane is synthesized around the outer surface of the endothelial cells.
|
|
|
|
|
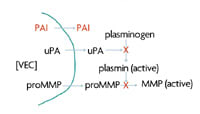
Figure 2. Anecortave acetate inhibits the proteolytic cascade involved in angiogenesis regardless of the initiating angiogenic stimulus. Source: Alcon Laboratories, Inc. |
In vitro data indicate that anecortave acetate inhibits the proliferation of cultured human VECs. It also inhibits the expression of uPA and matrix metalloproteinase 3 (MMP3), 2 extracellular proteases necessary for the migration of endothelial cells into tissue stroma during blood vessel growth.9 Additionally, it stimulates the production of plasminogen activator inhibitor-1 (PAI-1), a specific inhibitor of uPA activity.10 In a rat model of retinopathy of prematurity, retinal levels of PAI-1 and mRNA increased 6- to 9-fold in 24 to 72 hours following intravitreal injection of the novel angiostatic cortisene.11 With the activities of uPA and MMP3 inhibited by anecortave acetate, proteolysis necessary for cellular migration cannot occur and angiogenesis is inhibited (Figure 2).
PRECLINICAL EFFICACY OF ANECORTAVE ACETATE
In preclinical studies, anecortave acetate inhibited blood vessel growth in 12 in vivo and in vitro models. Intravitreal administration in a rat pup model of retinopathy of prematurity showed a 66% inhibition of retinal neovascularization and retinopathy.10 Additionally, retinal neovascularization in rats induced by constant low levels of light for up to 6 months was reduced ~30% when monthly subconjunctival injections were administered.8 In both the chick chorioallantoic membrane assay and the rabbit lipopolysaccharide-induced corneal pocket models of neovascularization, angiostatic activity was also observed with the compound.6 Topical ophthalmic administration of anecortave acetate suspensions across various dosages in a rabbit in vivo lipopolysaccharide-induced corneal neovascularization model resulted in >90% inhibition in the area of new corneal blood vessel growth.12
Inhibition of ocular tumors has also been reported with topical ocular administration of anecortave acetate. Following the transplantation of murine uveal melanoma cell lines (99E1) into the anterior segment of nude mice, the animals were subsequently treated with a 1% anecortave acetate suspension 3 times daily for up to 4 weeks. Tumors treated with the angiostatic cortisene grew significantly slower (P <0.025), and net tumor weight was less than one-third that of untreated tumors. Since anecortave acetate lacks the ability to halt tumor cell proliferation in vitro, these results are presumably due to restricted vascular growth in tumor cells.13
In contrast to other antiangiogenic agents like VEGF inhibitors and protein kinase C (PKC) inhibitors, anecortave acetate acts downstream and independent of the initiating angiogenic stimuli and, therefore, inhibits angiogenesis irrespective of the initiating angiogenic signal. This likely accounts for the broad-spectrum angiostatic nature of this compound and explains how the drug inhibits neovascularization in numerous tissues and species regardless of the inciting cause.
PRECLINICAL SAFETY OF ANECORTAVE ACETATE
Preclinical toxicity studies demonstrate the safety of anecortave acetate. Suspensions of 1% and 2% anecortave acetate were administered 3 times daily by topical administration onto rabbit eyes for 12 months. No treatment-related ocular or systemic toxicity was noted. Furthermore, no changes were observed in body or organ weights, histopathology, hematology, or biomicroscopy. Likewise, a single intravitreal injection of a 10% anecortave acetate suspension was devoid of adverse ocular or systemic toxicity, and a 4-week oral toxicity test in which rats were given 25 mg/kg to 225 mg/kg of the drug by oral gavage also failed to produce any adverse effects in serum chemistries, hematology, urinalysis, or histopathology.8 Salmonella typhimurium and Escherichia coli mammalian microsome reverse mutagenicity assays were negative, indicating that anecortave acetate is unlikely to have mutagenic actions. In preclinical studies with animals, there was no treatment-related ocular or systemic toxicity, and no adverse effects were observed in serum chemistries, hematology, urinalysis, or histopathology when the drug was administered as a transcleral posterior juxtascleral depot.
PHARMACOKINETICS AND DRUG ADMINISTRATION
Because of the rapid systemic metabolism of anecortave acetate, oral and systemic routes are not feasible. The oral bioavailability of anecortave acetate in rats and monkeys is less than 1% and therefore impractical for therapeutic oral use. Stable, nonirritating ocular suspensions have been formulated for both topical ocular and intraocular administration. Following topical ocular administration and subconjunctival injections of 14C-anecortave acetate, drug concentrations necessary to inhibit angiogenesis in the retina and choroid were not achieved.
Unlike topical administration, intravitreal injections of anecortave acetate do provide therapeutic levels of drug to the retina. However, routine injections into the globe are undesirable due to the potential for serious complications such as endophthalmitis and retinal detachment associated with intravitreal injections. Sub-Tenon's injections were found to provide therapeutic drug concentrations (>=0.1uM) in both the retina and choroid, but these injections were associated with too much variability with respect to needle placement. Data indicate that depot delivery of anecortave acetate is optimal only when the drug is placed in direct contact with the posterior scleral surface. When properly positioned juxtasclerally, the anecortave acetate depot provides therapeutic drug levels in the choroid and retina for 6 months. In rabbit studies utilizing sub-Tenon's needle technique to administer anecortave acetate, proper drug positioning was achieved only 73% of the time.
Poor drug positioning would result in an undesirably high incidence of treatment failures with sub-Tenon's injections due to low drug bioavailability; therefore, a specialized cannula and depot delivery technique were developed to provide accurate transscleral delivery of the drug to the retina and choroid. The specialized cannula assures accurate placement of the drug depot in direct contact with the sclera with proper positioning over the macula. Also, the insertion depth is limited to avoid damaging the optic nerve or posterior ciliary arteries, the insertion site is distant from the depot to avoid losing drug in periocular tissues, and the orifice on the device is positioned such that there is minimal potential for plugging by connective tissues. Thus far, this technique has been used in clinical trials to deliver >1700 juxtascleral depots in >700 patients with no serious adverse events.
CLINICAL TRIALS FOR ANECORTAVE ACETATE
C-98-03. Evaluation of the Safety and Efficacy of Anecortave Acetate Sterile Suspension (30 mg, 15 mg or 3 mg) versus Placebo for Inhibition of Neovascularization in Patients with Exudative AMD.
Beginning in 1999, 128 patients with exudative subfoveal AMD were enrolled in a double-masked, dose response study to test the efficacy of anecortave acetate. Patients were randomized to receive anecortave acetate (30 mg, 15 mg, or 3 mg) or placebo via posterior juxtascleral administration every 6 months for a total of 24 months. At the conclusion of each 6-month time interval, patients continued in the study if a masked examining ophthalmologist decided the patient might benefit from continued treatment.
Mean change from baseline in best-corrected logMAR visual acuity was the primary efficacy variable, with secondary efficacy outcomes being (a) the percentage of patients with preservation of vision (loss of less than 3 logMAR lines of visual acuity from baseline), (b) percentage of patients with clinically significant worsening of vision (loss of at least 3 logMAR lines from baseline), (c) percentage of patients with severe vision loss (loss of at least 6 logMAR lines from baseline), and (d) changes in CNV (total lesion area, total CNV, and total classic CNV).
|

|
|
|
Figure 3. Superiority of anecortave acetate 15 mg compared with placebo for long-term vision stabilization in all treated eyes. C-98-03 phaseII/III study. Source: Alcon Laboratories, Inc. |
|
Long-term study results confirm the efficacy of anecortave acetate 15 mg for maintaining vision and suppressing lesion growth in patients with exudative AMD.14 At month 24, anecortave acetate 15 mg was numerically superior to other dosages tested and statistically superior to placebo in preserving visual acuity (P ¾0.05), stabilizing vision (P ¾0.05), preventing severe vision loss (P ¾0.05), and inhibiting the growth of classic choroidal neovascularization (P ¾0.05) in eyes with all lesion types. Beginning at the 6-month timepoint and continuing throughout the entire 24-month study, anecortave acetate 15 mg was consistently more effective in stabilizing vision compared to placebo (Figure 3).
Anecortave acetate has been shown in vitro to inhibit endothelial cell proliferation and migration and has been shown clinically to inhibit CNV lesion growth and the formation of new blood vessels in the eye regardless of the inciting agent. This inhibition likely occurs whether the lesion is predominantly classic or minimally classic. The clinical efficacy observed in this study of a mixed population of AMD lesion types and sizes supports this observation.
C-00-07. An Evaluation of the Efficacy and Safety of Anecortave Acetate (30 mg and 15 mg) versus Placebo in Patients with Exudative Age-Related Macular Degeneration (AMD) Following Treatment with Photodynamic Therapy Using Visudyne.
A 6-month dose response phase II study was conducted to evaluate the safety and efficacy of 2 concentrations of anecortave acetate in combination with PDT with verteporfin in AMD patients with minimally classic and predominantly classic lesions. The primary efficacy endpoint was a mean change from baseline in logMAR visual acuity at month 6. The secondary objective was to demonstrate a dose response relationship between anecortave acetate administered as a posterior juxtascleral depot and the number of months until there was angiographic evidence for retreatment with PDT. Results from this study suggest a trend (not statistically significant) favoring both anecortave acetate concentrations tested (15 mg and 30 mg) combined with PDT over PDT alone (plus placebo) for both inhibition of lesion growth and preservation of vision. There were no drug interactions reported and no safety issues identified with coadministration of the 2 drugs.
SAFETY IN CLINICAL TRIALS
As of June 2004, >800 patients in the United States and the European Union have received at least 1 dose of either anecortave acetate or placebo in clinical trials. To date, no globe perforations, damage to the optic nerve, or damage to posterior ciliary arteries have been reported. Importantly, there is no evidence that anecortave acetate elevates intraocular pressure nor is there any indication that the novel cortisene accelerates cataract progression.14 Additionally, no treatment-related changes in blood chemistries, hematology, or urinalyses have occurred.15
In the clinical trials, no serious clinically relevant, treatment-related adverse events have been noted. Of adverse events reported, most (ocular pain, subconjunctival hemorrhage, pruritis, and ptosis) are commonly expected following posterior juxtascleral depot administration in patients with AMD and are not related to anecortave acetate treatment. Adverse events have been mild and transient and have occurred across all dosages tested, with similar incidence in anecortave acetate and placebo treatment groups, and have been seen in the fellow eye as well as the study eye.16
ADDITIONAL STUDIES
Exudative AMD
Four clinical efficacy and safety studies are now underway to evaluate anecortave acetate 15 mg for depot suspension for treatment of subfoveal CNV in patients with exudative AMD.
A pivotal study, C-01-99, designed to compare anecortave acetate 15 mg depot to PDT with verteporfin, has completed enrollment of 530 patients at >50 sites worldwide. Patients enrolled in this study were randomized 1:1 to either a posterior juxtascleral depot administration of anecortave acetate 15 mg depot every 6 months combined with a sham PDT treatment every 3 months per labeling guidelines, or to PDT every 3 months per labeling guidelines combined with a sham posterior juxtascleral administration every 6 months. The purpose of this masked, randomized study is to determine the safety and efficacy of posterior juxtascleral administrations of anecortave acetate 15 mg depot vs PDT for stabilization of visual acuity and inhibition of subfoveal CNV lesion growth.
Two smaller placebo controlled studies continue to enroll patients who are randomized to receive either anecortave acetate 15 mg for depot suspension or placebo. An open-label, rollover phase II study with anecortave acetate 15 mg depot is ongoing. It is designed to allow continued treatment of patients who exited from Clinical Study C-98-03 at its conclusion. Patients in this study receive posterior juxtascleral depot administrations of anecortave acetate 15 mg depot at 6-month intervals as needed.
Non-Exudative AMD
AART. Anecortave Acetate (15 mg or 30 mg) versus Sham for Treatment of Patients at Risk for Developing Choroidal Neovascularization (CNV) Due to Exudative Age-Related Macular Degeneration (AMD).
Based on the preclinical evidence that anecortave acetate is capable of preventing new blood vessel growth, the excellent clinical safety profile demonstrated in the completed and ongoing studies, and the efficacy data from 2 completed studies, a clinical trial has begun with anecortave acetate for the treatment of eyes with dry AMD.17 In this study, patients must have past or current exudative AMD in one eye and intermediate or large soft/confluent drusen with hyperpigmentation and no CNV or geographic atrophy in the study eye. The risk of the study eye developing CNV is ~50% (or higher) over 5 years.18 The purpose of the study is to determine if anecortave acetate can slow or prevent the progression of these at-risk eyes to CNV with its associated risk of clinically significant vision loss.
The excellent safety profile and demonstrated clinical efficacy of anecortave acetate makes it uniquely suited for this trial, which addresses an unmet medical need in a group of patients for which there are no approved treatment options. It also has the potential to reduce the risk of progression to CNV and subsequent vision loss in this patient population, which is expected to double over the next 20 years.
SUMMARY
Anecortave acetate is a unique synthetic cortisone with antiangiogenic properties that have been established in multiple experimental models of angiogenesis. It is currently being evaluated in clinical trials for patients with AMD. To date it has demonstrated an excellent safety record in >1700 posterior juxtascleral depot injections for >700 patients. No clinically relevant adverse events have been noted. Several ongoing clinical trials are evaluating 15 mg depot suspension for the treatment of subfoveal CNV in patients with wet AMD. Data from the pivotal C-01-99 trial comparing the 15 mg depot suspension with verteporfin PDT will be available in late 2004. Based on preclinical evidence that anecortave acetate is capable of preventing new blood vessel growth together with its safety profile, the new AART trial has begun. The objective of AART is to determine whether anecortave acetate can prevent the development of CNV and vision loss in AMD patients with unilateral drusen and good vision. This is a significant clinical trial in that it may prove to be effective in preserving useful central vision.
From Retina Service, Wills Eye Hospital and Thomas Jefferson University, Philadelphia, Pa. Dr. Ho receives funding for clinical trials from Alcon Laboratories, Inc.
REFERENCES
1. Swann PG, Lovie-Kitchin, JE. Age-related maculopathy: I. A review of its morphology and effects on visual function. Ophthalmic Physiol Opt. 1990; 10:149-158.
2. Lee PP, Feldman ZW, Osterman J, et al. Longitudinal prevalence of major eye diseases. Arch Ophthalmol. 2003; 121:1303-1310.
3. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal CNV in age-related macular degeneration with verteporfin. TAP Report 1. Arch Ophthalmol. 1999; 117:1329-1345.
4. Crum R, Szabo S, Folkman J. A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science. 1985; 230:1375-1378.
5. Blei F, Wilson EL, Mignatti P, et al. Mechanism of action of angiostatic steroids: suppression of plasminogen activator activity via stimulation of plasminogen activator inhibitor synthesis. J. Cell. Physiol. 1993;155:568-578.
6. McNatt LG, Weimer L, Yanni J, et al. Angiostatic activity of steroids in the chick embryo CAM and rabbit cornea models of neovascularization. J Ocul Pharmacol Thera. 1999;15:413-423.
7. Smith RE, Nozik RA. Uveitis: a clinical approach to diagnosis and management. In: The Nonspecific Treatment of Uveitis. 2nd ed. Baltimore, MD: Williams & Wilkins; 1988:51-76.
8. Clark AF. AL-3789: A novel ophthalmic angiostatic steroid. Exp Opin Invest Drugs. 1997;6:1867-1877.
9. DeFaller JM, Clark AF. A new pharmacological treatment for angiogenesis. In: Taylor HR, ed. Pterygium. The Hague, Netherlands: Kugler Publications; 2000:159-181.
10. Penn JS, Rajaratnam V, Collier RJ, Clark AF. The effect of an angiostatic steroid on neovascularization in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2001; 42:283-290.
11. BenEzra D, Griffin BW, Maftzir G, et al. Topical formulations of novel angiostatic steroids inhibit rabbit corneal neovascularization. Invest Ophthalmol Vis Sci .1997; 58:1954-1962
12. Clark AF, Mellon J, Li, XY, et al. Inhibition of intraocular tumor growth by topical application of the angiostatic steroid anecortave acetate. Invest Ophthalmol Vis Sci. 1999; 40:2158-2162.
13. Kaiser PK, Russell SR, Slakter JS, et al. Anecortave acetate monotherapy for treatment of subfoveal neovascularization in age-related macular degeneration (AMD): clinical outcomes at month 24. To be submitted for publication to Am J Ophthalmology.
14. Regillo CD, D'Amico DJ, Mieler WF, et al. Safety of anecortave acetate administered as a posterior juxtascleral injections in patients with CNV. Manuscript in preparation for submission to Survey of Ophthalmology.
15. D'Amico DJ, Goldbrg MF, Hudson H, et al. and the Anecortave Acetate Clinical Study Group. Anecortave acetate as monotherapy for the treatment of subfoveal lesions in patients with exudative age-related macular degeneration (AMD): interim (month 6) analysis of clinical safety and efficacy. Retina. 2003a; 23:14-23.
16. D'Amico DJ, Goldbrg MF, Hudson H, et al. and the Anecortave Acetate Clinical Study Group. Anecortave acetate as monotherapy for treatment of subfoveal neovascularization in age-related macular degeneration: Twelve month clinical outcomes. Ophthalmol. 2003b; 110:2372-2385.
17. Russell S, Slakter J, Ho A, et al. Anecortave acetate treatment of "dry" AMD to reduce risk of progression to "wet" AMD - the anecortave acetate risk reduction trial (AART). IOVS. 2004; 45:ARVO E-abstract 3134.
18. Macular Photocoagulation Study Group. Five-year follow-up of fellow eyes of patients with age-related macular degeneration and unilateral extrafoveal choroidal neovascularization. Arch. Ophthalmology. 1993; 111:1189-1199.








